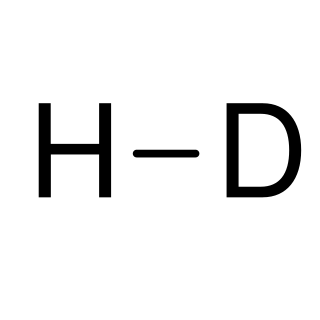Deuterium (or hydrogen-2, symbol 2
H
or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among every 6,420 atoms of hydrogen (see heavy water). Thus deuterium accounts for approximately 0.0156% by number (0.0312% by mass) of all the naturally occurring hydrogen in the oceans (i.e., 4.85×1013 tonnes of deuterium – mainly in form of HOD and only rarely in form of D2O – in 1.4×1018 tonnes of water), while protium accounts for 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water)
Chloroform, or trichloromethane, is an organic compound with the formula CHCl3 and a common solvent. It is a very volatile, colorless, strong-smelling, dense liquid produced on a large scale as a precursor to refrigerants and in turn PTFE. Chloroform is a trihalomethane that serves as a powerful anesthetic, euphoriant, anxiolytic, and sedative when inhaled or ingested. Chloroform was used as an anesthetic between the 19th century and the first half of the 20th century. It is miscible with many solvents but it is only very slightly soluble in water.

Dichloromethane is an organochlorine compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.

Semiheavy water is the result of replacing one of the protium in light water with deuterium. It exists whenever there is water with light hydrogen (protium, 1H) and deuterium (D or 2H) in the mix. This is because hydrogen atoms (hydrogen-1 and deuterium) are rapidly exchanged between water molecules. Water containing 50% H and 50% D in its hydrogen contains about 50% HDO and 25% each of H2O and D2O, in dynamic equilibrium. In regular water, about 1 molecule in 3,200 is HDO (one hydrogen in 6,400 is D). By comparison, heavy water D2O occurs at a proportion of about 1 molecule in 41 million (i.e., one in 6,4002). This makes semiheavy water far more common than "normal" heavy water.

Proton nuclear magnetic resonance is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen (H) is used, practically all the hydrogen consists of the isotope 1H.
Luca Turin is a biophysicist and writer with a long-standing interest in bioelectronics, the sense of smell, perfumery, and the fragrance industry.

An NMR tube is a thin glass walled tube used to contain samples in nuclear magnetic resonance spectroscopy. Typically NMR tubes come in 5 mm diameters but 10 mm and 3 mm samples are known. It is important that the tubes are uniformly thick and well-balanced to ensure that NMR tube spins at a regular rate, usually about 20 Hz in the NMR spectrometer.
Deuterated chloroform, also known as chloroform-d, is the organic compound with the formula CDCl3 or C2HCl3. Deuterated chloroform is a common solvent used in NMR spectroscopy. The properties of CDCl3 and ordinary CHCl3 (chloroform) are virtually identical.

Deuterated acetone ((CD3)2CO), also known as acetone-d6, is a form (called an isotopologue) of acetone (CH3)2CO in which the hydrogen atom ("H") is replaced with deuterium (heavy hydrogen) isotope ("D"). Deuterated acetone is a common solvent used in NMR spectroscopy.

Deuterated benzene (C6D6) is an isotopologue of benzene (C6H6) in which the hydrogen atom ("H") is replaced with deuterium (heavy hydrogen) isotope ("D").
Deuterated dimethylformamide ((CD3)2NCOD), also known as deuterated DMF, is an isotopologue of DMF ((CH3)2NCOH) in which the hydrogen atom ("H") is replaced with a deuterium isotope ("D"). Deuterated DMF is a relatively uncommon solvent used in NMR spectroscopy.

Deuterated ethanol (C2D5OD) is a form (called an isotopologue) of ethanol (C2H5OH) in which the hydrogen atom ("H") is replaced with deuterium (heavy hydrogen) isotope ("D"). Deuterated ethanol is an uncommon solvent used in NMR spectroscopy.

Deuterated methanol (CD3OD), is a form (called an isotopologue) of methanol (CH3OH) in which the hydrogen atoms ("H") are replaced with deuterium (heavy hydrogen) isotope ("D"). Deuterated methanol is a common solvent used in NMR spectroscopy.

Deuterated DMSO, also known as dimethyl sulfoxide-d6, is an isotopologue of dimethyl sulfoxide (DMSO, (CH3)2S=O)) with chemical formula ((CD3)2S=O) in which the hydrogen atoms ("H") are replaced with their isotope deuterium ("D"). Deuterated DMSO is a common solvent used in NMR spectroscopy.

Heavy isotope diet is the consumption of nutrients in which some atoms are replaced with their heavier non-radioactive isotopes, such as deuterium(2H) or heavy carbon (13C). Biomolecules that incorporate heavier isotopes give rise to more stable molecular structures under certain circumstances, which is hypothesized to increase resistance to damage associated with ageing or diseases.
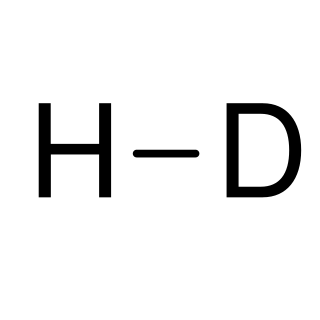
Hydrogen deuteride is an isotopologue of dihydrogen composed of two isotopes of hydrogen: the majority isotope 1H (protium) and 2H (deuterium). Its proper molecular formula is H2H, but for simplification, it is usually written as HD.
Trifluorotoluene is an organic compound with the formula of C6H5CF3. This colorless fluorocarbon is used as a specialty solvent in organic synthesis and an intermediate in the production of pesticides and pharmaceuticals.

Deuterated solvents are a group of compounds where one or more hydrogen atoms are substituted by deuterium atoms.
Deuterium NMR is NMR spectroscopy of deuterium, an isotope of hydrogen. Deuterium is an isotope with spin = 1, unlike hydrogen-1, which has spin = 1/2. The term deuteron NMR, in direct analogy to proton NMR, is also used. Deuterium NMR has a range of chemical shift similar to proton NMR but with poor resolution, due to the smaller magnitude of the magnetic dipole moment of the deuteron relative to the proton. It may be used to verify the effectiveness of deuteration: a deuterated compound will show a strong peak in deuterium NMR but not proton NMR.

A deuterated drug is a small molecule medicinal product in which one or more of the hydrogen atoms contained in the drug molecule have been replaced by its heavier stable isotope deuterium. Because of the kinetic isotope effect, deuterium-containing drugs may have significantly lower rates of metabolism, and hence a longer half-life.