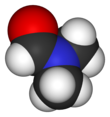
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, and in the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers. Some astrobiologists suggest that it may be an alternative to water as the main solvent in other forms of life.

Oxalyl chloride is an organic chemical compound with the formula Cl−C(=O)−C(=O)−Cl. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.

Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides/oxychlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.

DABCO (1,4-diazabicyclo[2.2.2]octane), also known as triethylenediamine or TEDA, is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis.
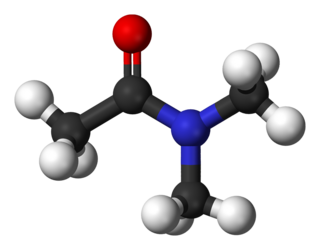
Dimethylacetamide (DMAc or DMA) is the organic compound with the formula CH3C(O)N(CH3)2. This colorless, water-miscible, high-boiling liquid is commonly used as a polar solvent in organic synthesis. DMA is miscible with most other solvents, although it is poorly soluble in aliphatic hydrocarbons.
The Vilsmeier–Haack reaction (also called the Vilsmeier reaction) is the chemical reaction of a substituted formamide (1) with phosphorus oxychloride and an electron-rich arene (3) to produce an aryl aldehyde or ketone (5):

Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.
The Duff reaction or hexamine aromatic formylation is a formylation reaction used in organic chemistry for the synthesis of benzaldehydes with hexamine as the formyl carbon source. The method is generally inefficient. The reaction is named after James Cooper Duff.
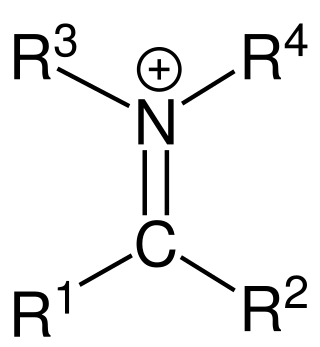
In organic chemistry, an iminium cation is a polyatomic ion with the general structure [R1R2C=NR3R4]+. They are common in synthetic chemistry and biology.

Phosphoryl chloride is a colourless liquid with the formula POCl3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters.
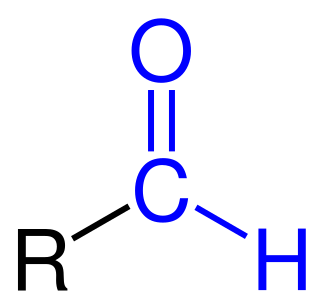
Formylation refers to any chemical processes in which a compound is functionalized with a formyl group (-CH=O). In organic chemistry, the term is most commonly used with regards to aromatic compounds. In biochemistry the reaction is catalysed by enzymes such as formyltransferases.

Crotonaldehyde is a chemical compound with the formula CH3CH=CHCHO. The compound is usually sold as a mixture of the E- and Z-isomers, which differ with respect to the relative position of the methyl and formyl groups. The E-isomer is more common (data given in Table is for the E-isomer). This lachrymatory liquid is moderately soluble in water and miscible in organic solvents. As an unsaturated aldehyde, crotonaldehyde is a versatile intermediate in organic synthesis. It occurs in a variety of foodstuffs, e.g. soybean oils.

In organic chemistry, carbonyl reduction is the conversion of any carbonyl group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketones, aldehydes, carboxylic acids, esters, amides, and acid halides - some of the most pervasive functional groups, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne is added to a carbonyl group to form an α-alkynyl alcohol.

Dimethylcarbamoyl chloride (DMCC) is a reagent for transferring a dimethylcarbamoyl group to alcoholic or phenolic hydroxyl groups forming dimethyl carbamates, usually having pharmacological or pesticidal activities. Because of its high toxicity and its carcinogenic properties shown in animal experiments and presumably also in humans, dimethylcarbamoyl chloride can only be used under stringent safety precautions.

3-Dimethylaminoacrolein is an organic compound with the formula Me2NC(H)=CHCHO. It is a pale yellow water-soluble liquid. The compound has a number of useful and unusual properties, e.g. it "causes a reversal of the hypnotic effect of morphine in mice" and has a "stimulating effect in humans".
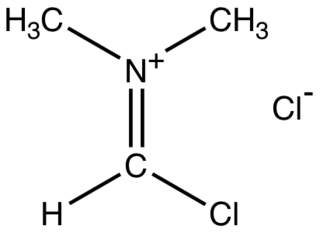
The Vilsmeier reagent is an organic compound with the formula [(CH3)2NCHCl]Cl. It is a salt consisting of the N,N-dimethyliminium cation ([(CH3)2N=CHCl]+) and chloride anion. Depending on the particular reaction, the anion can vary. In typical POCl3-based reactions, the anion is PO2Cl2−. The iminium cation [(CH3)2N=CHCl]+ is the reactive component of interest. This iminium species is a derivative of the imidoyl chloride CH3N=CHCl. Analogues of this particular reagent are generated when tertiary amides other than DMF are treated with POCl3.