
A plasticizer is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture.
The Composition C family is a family of related US-specified plastic explosives consisting primarily of RDX. All can be moulded by hand for use in demolition work and packed by hand into shaped charge devices. Variants have different proportions and plasticisers and include composition C-2, composition C-3, and composition C-4.

Adipic acid or hexanedioic acid is the organic compound with the formula (CH2)4(COOH)2. From an industrial perspective, it is the most important dicarboxylic acid: about 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon. Adipic acid otherwise rarely occurs in nature, but it is known as manufactured E number food additive E355. Salts and esters of adipic acid are known as adipates.

Pimelic acid is the organic compound with the formula HO2C(CH2)5CO2H. Pimelic acid is one CH
2 unit longer than a related dicarboxylic acid, adipic acid, a precursor to many polyesters and polyamides. However compared to adipic acid, pimelic acid is relatively small in importance industrially. Derivatives of pimelic acid are involved in the biosynthesis of the amino acid lysine and the vitamin biotin.

Potassium adipate is a compound with formula K2C6H8O4. It is a potassium salt and common source ingredient of adipic acid.
Sodium adipate is a compound with formula Na2C6H8O4. It is the sodium salt of adipic acid.
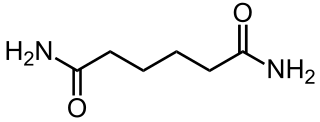
Adipamide is the organic compound with the formula (CH2CH2C(O)NH2)2. It is a white solid. The dominant commercial interest in adipamides is related to their presence in nylons.
Nylon 66 is a type of polyamide or nylon. It, and nylon 6, are the two most common for textile and plastic industries. Nylon 66 is made of two monomers each containing 6 carbon atoms, hexamethylenediamine and adipic acid, which give nylon 66 its name. Aside from its superior physical characteristics, nylon 66 is attractive because its precursors are inexpensive.
PBAT is a biodegradable random copolymer, specifically a copolyester of adipic acid, 1,4-butanediol and terephthalic acid. PBAT is produced by many different manufacturers and may be known by the brand names ecoflex, Wango,Ecoworld, Eastar Bio, and Origo-Bi. It is also called poly(butylene adipate-co-terephthalate) and sometimes polybutyrate-adipate-terephthalate or even just "polybutyrate". It is generally marketed as a fully biodegradable alternative to low-density polyethylene, having many similar properties including flexibility and resilience, allowing it to be used for many similar uses such as plastic bags and wraps. It is depicted as a block co-polymer here due to the common synthetic method of first synthesizing two copolymer blocks and then combining them. However, it is important to note that the actual structure of the polymer is a random co-polymer of the blocks shown.

Ammonium adipate is a compound with formula (NH4)2(C4H8(COO)2). It is the ammonium salt of adipic acid. It is used as a food additive and has the E number E359.
Dibasic ester or DBE is an ester of a dicarboxylic acid. Depending on the application, the alcohol may be methanol or higher molecular weight monoalcohols.

Acetylated distarch adipate (E1422), is a starch that is treated with acetic anhydride and adipic acid anhydride to resist high temperatures. It is used in foods as a bulking agent, stabilizer and a thickener.

Docusate is the common chemical and pharmaceutical name of the anion bis(2-ethylhexyl) sulfosuccinate, also commonly called dioctyl sulfosuccinate (DOSS). It is on the World Health Organization's List of Essential Medicines. Salts of this anion, especially docusate sodium, are widely used in medicine as laxatives and as stool softeners, by mouth or rectally. In 2020, it was the 163rd most commonly prescribed medication in the United States, with more than 3 million prescriptions. Some studies claim that docusate is not more effective than a placebo for improving constipation. Other docusate salts with medical use include those of calcium and potassium.
Commodity chemicals are a group of chemicals that are made on a very large scale to satisfy global markets. The average prices of commodity chemicals are regularly published in the chemical trade magazines and web sites such as Chemical Week and ICIS. There have been several studies of the scale and complexity of this market for example in the USA.
Bis(2-ethylhexyl) adipate or DEHA or DOA is an organic compound with the formula (CH2CH2CO2C8H17)2. It is the diester of 2-ethylhexanol and adipic acid. It is a colorless oily liquid.
The molecular formula C22H42O4 (molar mass: 370.56 g/mol) may refer to:
1,6-Hexanediol is an organic compound with the formula (CH2CH2CH2OH)2. It is a colorless water-soluble solid.

Poly(ethylene adipate) or PEA is an aliphatic polyester. It is most commonly synthesized from a polycondensation reaction between ethylene glycol and adipic acid. PEA has been studied as it is biodegradable through a variety of mechanisms and also fairly inexpensive compared to other polymers. Its lower molecular weight compared to many polymers aids in its biodegradability.

Dimethyl adipate is the organic compound with the formula (CH2CH2CO2CH3)2. It is a colorless oily liquid. Although the main commercial interest in adipates is related to the production of nylons, this diester is used as a plasticizer, a solvent for paint stripping and resins, and a pigment dispersant.









