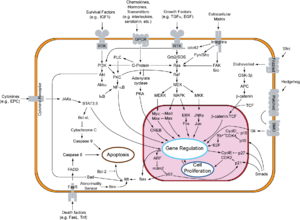
Paracrine signaling is a form of cell signaling, a type of cellular communication in which a cell produces a signal to induce changes in nearby cells, altering the behaviour of those cells. Signaling molecules known as paracrine factors diffuse over a relatively short distance, as opposed to cell signaling by endocrine factors, hormones which travel considerably longer distances via the circulatory system; juxtacrine interactions; and autocrine signaling. Cells that produce paracrine factors secrete them into the immediate extracellular environment. Factors then travel to nearby cells in which the gradient of factor received determines the outcome. However, the exact distance that paracrine factors can travel is not certain.
The Wnt signaling pathways are a group of signal transduction pathways which begin with proteins that pass signals into a cell through cell surface receptors. The name Wnt is a portmanteau created from the names Wingless and Int-1. Wnt signaling pathways use either nearby cell-cell communication (paracrine) or same-cell communication (autocrine). They are highly evolutionarily conserved in animals, which means they are similar across animal species from fruit flies to humans.
Adenomatous polyposis coli (APC) also known as deleted in polyposis 2.5 (DP2.5) is a protein that in humans is encoded by the APC gene. The APC protein is a negative regulator that controls beta-catenin concentrations and interacts with E-cadherin, which are involved in cell adhesion. Mutations in the APC gene may result in colorectal cancer.
Frzb is a Wnt-binding protein especially important in embryonic development. It is a competitor for the cell-surface G-protein receptor Frizzled.
Catenin beta-1, also known as beta-catenin (β-catenin), is a protein that in humans is encoded by the CTNNB1 gene.

Frizzled is a family of atypical G protein-coupled receptors that serve as receptors in the Wnt signaling pathway and other signaling pathways. When activated, Frizzled leads to activation of Dishevelled in the cytosol.

Glycogen synthase kinase-3 beta, (GSK-3 beta), is an enzyme that in humans is encoded by the GSK3B gene. In mice, the enzyme is encoded by the Gsk3b gene. Abnormal regulation and expression of GSK-3 beta is associated with an increased susceptibility towards bipolar disorder.

Axin-1 is a protein that in humans is encoded by the AXIN1 gene.

Ras GTPase-activating-like protein IQGAP1 (IQGAP1) also known as p195 is a ubiquitously expressed protein that in humans is encoded by the IQGAP1 gene. IQGAP1 is a scaffold protein involved in regulating various cellular processes ranging from organization of the actin cytoskeleton, transcription, and cellular adhesion to regulating the cell cycle.

Segment polarity protein dishevelled homolog DVL-1 is a protein that in humans is encoded by the DVL1 gene.

Secreted frizzled-related protein 1, also known as SFRP1, is a protein which in humans is encoded by the SFRP1 gene.

Low-density lipoprotein receptor-related protein 6 is a protein that in humans is encoded by the LRP6 gene. LRP6 is a key component of the LRP5/LRP6/Frizzled co-receptor group that is involved in canonical Wnt pathway.

Protein Wnt-3a is a protein that in humans is encoded by the WNT3A gene.

Segment polarity protein dishevelled homolog DVL-2 is a protein that in humans is encoded by the DVL2 gene.

Segment polarity protein dishevelled homolog DVL-3 is a protein that in humans is encoded by the DVL3 gene.
Naked cuticle 1 (NKD1) is a human gene that encodes the protein Nkd1, a member of the Naked cuticle (Nkd) family of proteins that regulate the Wnt signaling pathway. Insects typically have a single Nkd gene, whereas there are two Nkd genes, Nkd1 and Nkd2, in most vertebrates studied to date. Nkd1 binds to the Dishevelled (Dvl) family of proteins. Specific truncating NKD1 mutations identified in DNA mismatch repair deficient colon cancer that disrupt Nkd1/Dvl binding implicate these mutations as a cause of increased Wnt signaling in approximately 1% of human colon cancer, the majority of which have increased Wnt signaling due to mutations the adenomatous polyposis coli (APC), AXIN2, or rarely the beta-catenin genes.

In molecular biology, the DEP domain is a globular protein domain of about 80 amino acids that is found in over 50 proteins involved in G-protein signalling pathways. It was named after the three proteins it was initially found in:
Kang-Yell Choi is a professor of biotechnology at Yonsei University, and has a joint appointment position as a CEO of CK Regeon Inc. in Seoul, Korea. He has been performing researches related to cellular signaling, especially for the Wnt/β-catenin pathway involving various pathophysiologies. Choi has been leading the Translational Research Center for Protein Function Control (TRCP), a Korean government supported drug development institute, as a director for 10 years. Choi has been carrying out R&D to develop agents controlling the Wnt/β-catenin signaling pathway. Choi's main interest is development of the agents to treat intractable diseases that suppress tissue regeneration system through overexpression of CXXC5 and subsequent suppression of the Wnt/β-catenin signaling.
Protein Transduction Domain-fused Dishevelled Binding Motif (PTD-DBM) is a man-made peptide which interacts with the mechanism of the hair loss linked endogenous protein, CXXC5, which is a negative feedback regulator of the Wnt/β-catenin pathway. Application of the peptide to bald laboratory mice resulted in new hair follicle growth.

Dishevelled binding antagonist of beta catenin 1 is a protein that in humans is encoded by the DACT1 gene. Dact1 was originally described in 2002 as a negative regulator of Wnt signaling by binding and destabilizing Dishevelled. More recent investigation into the molecular function of Dact1 has identified its principle role in the cell as a scaffold to generate membrane-less biomolecular condensates through liquid-liquid phase separation. Mutations in the phase-separating regions of Dact1 lead to Townes-Brock Syndrome 2 while its overexpression is associated with bone metastasis.