
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects to float on a water surface without becoming even partly submerged.

Capillary action is the process of a liquid flowing in a narrow space in opposition to or at least without the assistance of any external forces like gravity.
Electrowetting is the modification of the wetting properties of a surface with an applied electric field.
In physics, Washburn's equation describes capillary flow in a bundle of parallel cylindrical tubes; it is extended with some issues also to imbibition into porous materials. The equation is named after Edward Wight Washburn; also known as Lucas–Washburn equation, considering that Richard Lucas wrote a similar paper three years earlier, or the Bell-Cameron-Lucas-Washburn equation, considering J.M. Bell and F.K. Cameron's discovery of the form of the equation in 1906.

Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with the first one. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces. There are two types of wetting: non-reactive wetting and reactive wetting.

The Marangoni effect is the mass transfer along an interface between two phases due to a gradient of the surface tension. In the case of temperature dependence, this phenomenon may be called thermo-capillary convection.
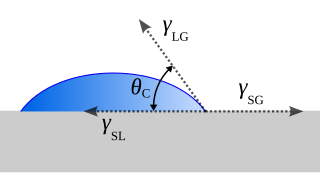
The contact angle is the angle between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface and the tangent on the solid–liquid interface at their intersection. It quantifies the wettability of a solid surface by a liquid via the Young equation.
The name electrospray is used for an apparatus that employs electricity to disperse a liquid or for the fine aerosol resulting from this process. High voltage is applied to a liquid supplied through an emitter. Ideally the liquid reaching the emitter tip forms a Taylor cone, which emits a liquid jet through its apex. Varicose waves on the surface of the jet lead to the formation of small and highly charged liquid droplets, which are radially dispersed due to Coulomb repulsion.
In fluid statics, capillary pressure is the pressure between two immiscible fluids in a thin tube, resulting from the interactions of forces between the fluids and solid walls of the tube. Capillary pressure can serve as both an opposing or driving force for fluid transport and is a significant property for research and industrial purposes. It is also observed in natural phenomena.

In fluid dynamics, the Plateau–Rayleigh instability, often just called the Rayleigh instability, explains why and how a falling stream of fluid breaks up into smaller packets with the same volume but less surface area. It is related to the Rayleigh–Taylor instability and is part of a greater branch of fluid dynamics concerned with fluid thread breakup. This fluid instability is exploited in the design of a particular type of ink jet technology whereby a jet of liquid is perturbed into a steady stream of droplets.
In physics, the Young–Laplace equation is an algebraic equation that describes the capillary pressure difference sustained across the interface between two static fluids, such as water and air, due to the phenomenon of surface tension or wall tension, although use of the latter is only applicable if assuming that the wall is very thin. The Young–Laplace equation relates the pressure difference to the shape of the surface or wall and it is fundamentally important in the study of static capillary surfaces. It is a statement of normal stress balance for static fluids meeting at an interface, where the interface is treated as a surface :

The capillary length or capillary constant, is a length scaling factor that relates gravity and surface tension. It is a fundamental physical property that governs the behavior of menisci, and is found when body forces (gravity) and surface forces are in equilibrium.

The Laplace pressure is the pressure difference between the inside and the outside of a curved surface that forms the boundary between two fluid regions. The pressure difference is caused by the surface tension of the interface between liquid and gas, or between two immiscible liquids.

In materials science and biology, capillary condensation is the "process by which multilayer adsorption from the vapor [phase] into a porous medium proceeds to the point at which pore spaces become filled with condensed liquid from the vapor [phase]." The unique aspect of capillary condensation is that vapor condensation occurs below the saturation vapor pressure, Psat, of the pure liquid. This result is due to an increased number of van der Waals interactions between vapor phase molecules inside the confined space of a capillary. Once condensation has occurred, a meniscus immediately forms at the liquid-vapor interface which allows for equilibrium below the saturation vapor pressure. Meniscus formation is dependent on the surface tension of the liquid and the shape of the capillary, as shown by the Young-Laplace equation. As with any liquid-vapor interface involving a meniscus, the Kelvin equation provides a relation for the difference between the equilibrium vapor pressure and the saturation vapor pressure. A capillary does not necessarily have to be a tubular, closed shape, but can be any confined space with respect to its surroundings.
The spinning drop method or rotating drop method is one of the methods used to measure interfacial tension. Measurements are carried out in a rotating horizontal tube which contains a dense fluid. A drop of a less dense liquid or a gas bubble is placed inside the fluid. Since the rotation of the horizontal tube creates a centrifugal force towards the tube walls, the liquid drop will start to deform into an elongated shape; this elongation stops when the interfacial tension and centrifugal forces are balanced. The surface tension between the two liquids can then be derived from the shape of the drop at this equilibrium point. A device used for such measurements is called a “spinning drop tensiometer”.
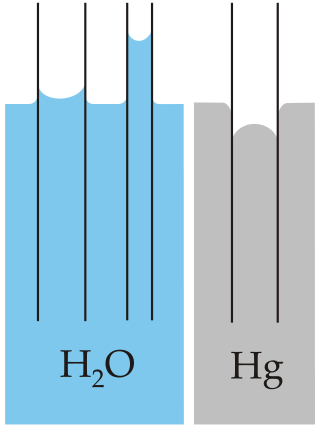
Jurin's law, or capillary rise, is the simplest analysis of capillary action—the induced motion of liquids in small channels—and states that the maximum height of a liquid in a capillary tube is inversely proportional to the tube's diameter. Capillary action is one of the most common fluid mechanical effects explored in the field of microfluidics. Jurin's law is named after James Jurin, who discovered it between 1718 and 1719. His quantitative law suggests that the maximum height of liquid in a capillary tube is inversely proportional to the tube's diameter. The difference in height between the surroundings of the tube and the inside, as well as the shape of the meniscus, are caused by capillary action. The mathematical expression of this law can be derived directly from hydrostatic principles and the Young–Laplace equation. Jurin's law allows the measurement of the surface tension of a liquid and can be used to derive the capillary length.
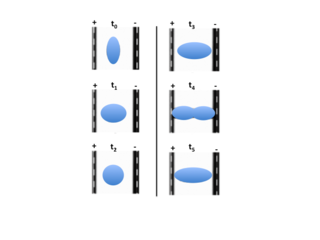
Electrohydrodynamic droplet deformation is a phenomenon that occurs when liquid droplets suspended in a second immiscible liquid are exposed to an oscillating electric field. Under these conditions, the droplet will periodically deform between prolate and oblate ellipsoidal shapes. The characteristic frequency and magnitude of the deformation is determined by a balance of electrodynamic, hydrodynamic, and capillary stresses acting on the droplet interface. This phenomenon has been studied extensively both mathematically and experimentally because of the complex fluid dynamics that occur. Characterization and modulation of electrodynamic droplet deformation is of particular interest for engineering applications because of the growing need to improve the performance of complex industrial processes(e.g. two-phase cooling, crude oil demulsification). The primary advantage of using oscillatory droplet deformation to improve these engineering processes is that the phenomenon does not require sophisticated machinery or the introduction of heat sources. This effectively means that improving performance via oscillatory droplet deformation is simple and in no way diminishes the effectiveness of the existing engineering system.

Elasto-capillarity is the ability of capillary force to deform an elastic material. From the viewpoint of mechanics, elastocapillarity phenomena essentially involve competition between the elastic strain energy in the bulk and the energy on the surfaces/interfaces. In the modeling of these phenomena, some challenging issues are, among others, the exact characterization of energies at the micro scale, the solution of strongly nonlinear problems of structures with large deformation and moving boundary conditions, and instability of either solid structures or droplets/films.The capillary forces are generally negligible in the analysis of macroscopic structures but often play a significant role in many phenomena at small scales.
Capillary breakup rheometry is an experimental technique used to assess the extensional rheological response of low viscous fluids. Unlike most shear and extensional rheometers, this technique does not involve active stretch or measurement of stress or strain but exploits only surface tension to create a uniaxial extensional flow. Hence, although it is common practice to use the name rheometer, capillary breakup techniques should be better addressed to as indexers.
The raindrop size distribution (DSD), or granulometry of rain, is the distribution of the number of raindrops according to their diameter (D). Three processes account for the formation of drops: water vapor condensation, accumulation of small drops on large drops and collisions between sizes. According to the time spent in the cloud, the vertical movement in it and the ambient temperature, the drops that have a very varied history and a distribution of diameters from a few micrometers to a few millimeters.










































