
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.

Ozone is an inorganic molecule with the chemical formula O
3. It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O
2, breaking down in the lower atmosphere to O
2 (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation.

Hazardous waste is waste that has substantial or potential threats to public health or the environment. Hazardous waste is a type of dangerous goods. They usually have one or more of the following hazardous traits: ignitability, reactivity, corrosivity, toxicity. Listed hazardous wastes are materials specifically listed by regulatory authorities as hazardous wastes which are from non-specific sources, specific sources, or discarded chemical products. Hazardous wastes may be found in different physical states such as gaseous, liquids, or solids. A hazardous waste is a special type of waste because it cannot be disposed of by common means like other by-products of our everyday lives. Depending on the physical state of the waste, treatment and solidification processes might be required.
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities are on the right-hand side with a plus sign between the entities in both the reactants and the products, and an arrow that points towards the products to show the direction of the reaction. The chemical formulas may be symbolic, structural, or intermixed. The coefficients next to the symbols and formulas of entities are the absolute values of the stoichiometric numbers. The first chemical equation was diagrammed by Jean Beguin in 1615.

A landfill site, also known as a tip, dump, rubbish dump, garbage dump, trash dump, or dumping ground, is a site for the disposal of waste materials. Landfill is the oldest and most common form of waste disposal, although the systematic burial of the waste with daily, intermediate and final covers only began in the 1940s. In the past, refuse was simply left in piles or thrown into pits; in archeology this is known as a midden.

Toxic waste is any unwanted material in all forms that can cause harm. Mostly generated by industry, consumer products like televisions, computers, and phones contain toxic chemicals that can pollute the air and contaminate soil and water. Disposing of such waste is a major public health issue.

Ethylene oxide is an organic compound with the formula C2H4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of a silver catalyst.

The Agency for Toxic Substances and Disease Registry (ATSDR) is a federal public health agency within the United States Department of Health and Human Services. The agency focuses on minimizing human health risks associated with exposure to hazardous substances. It works closely with other federal, state, and local agencies; tribal governments; local communities; and healthcare providers. Its mission is to "Serve the public through responsive public health actions to promote healthy and safe environments and prevent harmful exposures." ATSDR was created as an advisory, nonregulatory agency by the Superfund legislation and was formally organized in 1985.

Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is usually handled as an aqueous solution. It is commonly used as a bleach. More recent developments have extended its applications in food processing and as a disinfectant.
In environmental chemistry, the chemical oxygen demand (COD) is an indicative measure of the amount of oxygen that can be consumed by reactions in a measured solution. It is commonly expressed in mass of oxygen consumed over volume of solution which in SI units is milligrams per litre (mg/L). A COD test can be used to easily quantify the amount of organics in water. The most common application of COD is in quantifying the amount of oxidizable pollutants found in surface water or wastewater. COD is useful in terms of water quality by providing a metric to determine the effect an effluent will have on the receiving body, much like biochemical oxygen demand (BOD).

Environmental chemistry is the scientific study of the chemical and biochemical phenomena that occur in natural places. It should not be confused with green chemistry, which seeks to reduce potential pollution at its source. It can be defined as the study of the sources, reactions, transport, effects, and fates of chemical species in the air, soil, and water environments; and the effect of human activity and biological activity on these. Environmental chemistry is an interdisciplinary science that includes atmospheric, aquatic and soil chemistry, as well as heavily relying on analytical chemistry and being related to environmental and other areas of science.
Green chemistry, similar to sustainable chemistry or circular chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. While environmental chemistry focuses on the effects of polluting chemicals on nature, green chemistry focuses on the environmental impact of chemistry, including lowering consumption of nonrenewable resources and technological approaches for preventing pollution.
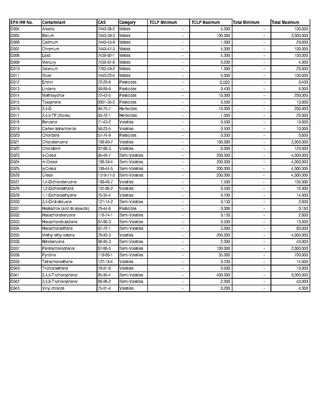
Toxicity characteristic leaching procedure (TCLP) is a soil sample extraction method for chemical analysis employed as an analytical method to simulate leaching through a landfill. The testing methodology is used to determine if a waste is characteristically hazardous, i.e., classified as one of the "D" listed wastes by the U.S. Environmental Protection Agency (EPA). The extract is analyzed for substances appropriate to the protocol.

Total organic carbon (TOC) is an analytical parameter representing the concentration of organic carbon in a sample. TOC determinations are made in a variety of application areas. For example, TOC may be used as a non-specific indicator of water quality, or TOC of source rock may be used as one factor in evaluating a petroleum play. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.

An environmental hazard are those hazards where the effects are seen in biomes or ecosystems rather than directly on living organisms. Environmental hazards can be a substance, state or event which has the potential to threaten the surrounding natural environment or adversely affect people's health. Well known examples include oil spills, water pollution, slash and burn deforestation, air pollution, and ground fissures. It can include any single or combination of toxic chemical, biological, or physical agents in the environment, resulting from human activities or natural processes. These agents may impact the health of exposed subjects, including pollutants such as heavy metals, pesticides, biological contaminants, toxic waste, industrial and home chemicals.
Selective catalytic reduction (SCR) means of converting nitrogen oxides, also referred to as NO
x with the aid of a catalyst into diatomic nitrogen, and water. A reductant, typically anhydrous ammonia, aqueous ammonia, or a urea solution, is added to a stream of flue or exhaust gas and is reacted onto a catalyst. As the reaction drives toward completion, nitrogen, and carbon dioxide, in the case of urea use, are produced.

Barium chlorate, Ba(ClO3)2, is the barium salt of chloric acid. It is a white crystalline solid, and like all soluble barium compounds, irritant and toxic. It is sometimes used in pyrotechnics to produce a green color. It also finds use in the production of chloric acid.

Under United States environmental policy, hazardous waste is a waste that has the potential to:
The environmental effect of pharmaceuticals and personal care products (PPCPs) is being investigated since at least the 1990s. PPCPs include substances used by individuals for personal health or cosmetic reasons and the products used by agribusiness to boost growth or health of livestock. More than twenty million tons of PPCPs are produced every year. The European Union has declared pharmaceutical residues with the potential of contamination of water and soil to be "priority substances".[3]
The environmental impact of concrete, its manufacture, and its applications, are complex, driven in part by direct impacts of construction and infrastructure, as well as by CO2 emissions; between 4-8% of total global CO2 emissions come from concrete. Many depend on circumstances. A major component is cement, which has its own environmental and social impacts and contributes largely to those of concrete.














