
A gamete is a haploid cell that fuses with another haploid cell during fertilization in organisms that reproduce sexually. Gametes are an organism's reproductive cells, also referred to as sex cells. The name gamete was introduced by the German cytologist Eduard Strasburger.

A gametophyte is one of the two alternating multicellular phases in the life cycles of plants and algae. It is a haploid multicellular organism that develops from a haploid spore that has one set of chromosomes. The gametophyte is the sexual phase in the life cycle of plants and algae. It develops sex organs that produce gametes, haploid sex cells that participate in fertilization to form a diploid zygote which has a double set of chromosomes. Cell division of the zygote results in a new diploid multicellular organism, the second stage in the life cycle known as the sporophyte. The sporophyte can produce haploid spores by meiosis that on germination produce a new generation of gametophytes.

Sex is the trait that determines whether a sexually reproducing organism produces male or female gametes. During sexual reproduction, a male and a female gamete fuse to form a zygote, which develops into an offspring that inherits traits from each parent. By convention, organisms that produce smaller, more mobile gametes are called male, while organisms that produce produce larger, non-mobile gametes are called female. An organism that produces both types of gamete is hermaphrodite.

A sex-determination system is a biological system that determines the development of sexual characteristics in an organism. Most organisms that create their offspring using sexual reproduction have two common sexes and a few less common intersex variations.

A sporophyte is the diploid multicellular stage in the life cycle of a plant or alga which produces asexual spores. This stage alternates with a multicellular haploid gametophyte phase.

Sexual differentiation is the process of development of the sex differences between males and females from an undifferentiated zygote. Sex determination is often distinct from sex differentiation; sex determination is the designation for the development stage towards either male or female, while sex differentiation is the pathway towards the development of the phenotype.

Plant reproductive morphology is the study of the physical form and structure of those parts of plants directly or indirectly concerned with sexual reproduction.
In biology, gonochorism is a sexual system where there are two sexes and each individual organism is either male or female. The term gonochorism is usually applied in animal species, the vast majority of which are gonochoric.
Dioecy is a characteristic of certain species that have distinct unisexual individuals, each producing either male or female gametes, either directly or indirectly. Dioecious reproduction is biparental reproduction. Dioecy has costs, since only the female part of the population directly produces offspring. It is one method for excluding self-fertilization and promoting allogamy (outcrossing), and thus tends to reduce the expression of recessive deleterious mutations present in a population. Plants have several other methods of preventing self-fertilization including, for example, dichogamy, herkogamy, and self-incompatibility.

Sequential hermaphroditism is one of the two types of hermaphroditism, the other type being simultaneous hermaphroditism. It occurs when the organism's sex changes at some point in its life. In particular, a sequential hermaphrodite produces eggs and sperm at different stages in life. Sequential hermaphroditism occurs in many fish, gastropods, and plants. Species that can undergo these changes do so as a normal event within their reproductive cycle, usually cued by either social structure or the achievement of a certain age or size. In some species of fish, sequential hermaphroditism is much more common than simultaneous hermaphroditism.
Sex allocation is the allocation of resources to male versus female reproduction in sexual species. Sex allocation theory tries to explain why many species produce equal number of males and females.
Plant reproduction is the production of new offspring in plants, which can be accomplished by sexual or asexual reproduction. Sexual reproduction produces offspring by the fusion of gametes, resulting in offspring genetically different from either parent. Asexual reproduction produces new individuals without the fusion of gametes, resulting in clonal plants that are genetically identical to the parent plant and each other, unless mutations occur.

Sex chromosomes are chromosomes that carry the genes that determine the sex of an individual. The human sex chromosomes are a typical pair of mammal allosomes. They differ from autosomes in form, size, and behavior. Whereas autosomes occur in homologous pairs whose members have the same form in a diploid cell, members of an allosome pair may differ from one another.

Ceratopteris is the only genus among homosporous ferns that is exclusively aquatic. It is pan-tropical and classified in the Parkerioideae subfamily of the family Pteridaceae.

A hermaphrodite is a sexually reproducing organism that produces both male and female gametes. Animal species in which individuals are of different sexes, either male or female but not both, are gonochoric, which is the opposite of hermaphroditic.
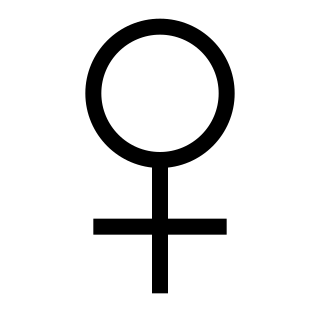
An organism's sex is female if it produces the ovum, the type of gamete that fuses with the male gamete during sexual reproduction.

Sexual reproduction is a type of reproduction that involves a complex life cycle in which a gamete with a single set of chromosomes combines with another gamete to produce a zygote that develops into an organism composed of cells with two sets of chromosomes (diploid). This is typical in animals, though the number of chromosome sets and how that number changes in sexual reproduction varies, especially among plants, fungi, and other eukaryotes.

Ceratopteris richardii is a fern species belonging to the genus Ceratopteris, one of only two genera of the subfamily Parkerioideae of the family Pteridaceae. It is one of several genera of ferns adapted to an aquatic existence. C. richardii was previously regarded as being part of the species Ceratopteris thalictroides.
Antheridiogens are a class of chemicals secreted by fern gametophytes that have "been shown to influence production of male gametangia and thus mating systems in a large number of terrestrial fern species". Antheridiogens are only observed in homosporous fern species, as all gametophytes are potentially bisexual.

Simultaneous hermaphroditism is one of the two types of hermaphroditism, the other type being sequential hermaphroditism. In this form of hermaphroditism an individual has sex organs of both sexes and can produce both gamete types even in the same breeding season.
















