
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration.

Titration is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte. A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of analyte to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the titration volume.
A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually. Hence, a pH indicator is a chemical detector for hydronium ions (H3O+) or hydrogen ions (H+) in the Arrhenius model. Normally, the indicator causes the color of the solution to change depending on the pH. Indicators can also show change in other physical properties; for example, olfactory indicators show change in their odor. The pH value of a neutral solution is 7.0 at 25°C (standard laboratory conditions). Solutions with a pH value below 7.0 are considered acidic and solutions with pH value above 7.0 are basic. Since most naturally occurring organic compounds are weak electrolytes, such as carboxylic acids and amines, pH indicators find many applications in biology and analytical chemistry. Moreover, pH indicators form one of the three main types of indicator compounds used in chemical analysis. For the quantitative analysis of metal cations, the use of complexometric indicators is preferred, whereas the third compound class, the redox indicators, are used in redox titrations (titrations involving one or more redox reactions as the basis of chemical analysis).

Karl Fischer titration is a classic titration method in chemical analysis that uses coulometric or volumetric titration to determine trace amounts of water in a sample. It was invented in 1935 by the German chemist Karl Fischer. Today, the titration is done with an automated Karl Fischer titrator.

In chemistry, neutralization or neutralisation is a chemical reaction in which acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants.
A redox titration is a type of titration based on a redox reaction between the analyte and titrant. It may involve the use of a redox indicator and/or a potentiometer. A common example of a redox titration is treating a solution of iodine with a reducing agent to produce iodide using a starch indicator to help detect the endpoint. Iodine (I2) can be reduced to iodide (I−) by, say, thiosulfate (S2O2−3, and when all iodine is spent the blue colour disappears. This is called an iodometric titration.
Complexometric titration is a form of volumetric analysis in which the formation of a colored complex is used to indicate the end point of a titration. Complexometric titrations are particularly useful for the determination of a mixture of different metal ions in solution. An indicator capable of producing an unambiguous color change is usually used to detect the end-point of the titration. Complexometric titration are those reactions where a simple ion is transformed into a complex ion and the equivalence point is determined by using metal indicators or electrometrically.

An acid–base titration is a method of quantitative analysis for determining the concentration of an acid or base by exactly neutralizing it with a standard solution of base or acid having known concentration. A pH indicator is used to monitor the progress of the acid–base reaction. If the acid dissociation constant (pKa) of the acid or base dissociation constant (pKb) of base in the analyte solution is known, its solution concentration (molarity) can be determined. Alternately, the pKa can be determined if the analyte solution has a known solution concentration by constructing a titration curve.
Coulometry determines the amount of matter transformed during an electrolysis reaction by measuring the amount of electricity consumed or produced. It can be used for precision measurements of charge, and the amperes even used to have a coulometric definition. However, today coulometry is mainly used for analytical applications. Coulometry is a group of techniques in analytical chemistry. It is named after Charles-Augustin de Coulomb.
Amperometric titration refers to a class of titrations in which the equivalence point is determined through measurement of the electric current produced by the titration reaction. It is a form of quantitative analysis.
Iodometry, known as iodometric titration, is a method of volumetric chemical analysis, a redox titration where the appearance or disappearance of elementary iodine indicates the end point.

Wet chemistry is a form of analytical chemistry that uses classical methods such as observation to analyze materials. It is called wet chemistry since most analyzing is done in the liquid phase. Wet chemistry is also called bench chemistry since many tests are performed at lab benches.
In analytical chemistry, quantitative analysis is the determination of the absolute or relative abundance of one, several or all particular substance(s) present in a sample.
A thermometric titration is one of a number of instrumental titration techniques where endpoints can be located accurately and precisely without a subjective interpretation on the part of the analyst as to their location. Enthalpy change is arguably the most fundamental and universal property of chemical reactions, so the observation of temperature change is a natural choice in monitoring their progress. It is not a new technique, with possibly the first recognizable thermometric titration method reported early in the 20th century. In spite of its attractive features, and in spite of the considerable research that has been conducted in the field and a large body of applications that have been developed; it has been until now an under-utilized technique in the critical area of industrial process and quality control. Automated potentiometric titration systems have pre-dominated in this area since the 1970s. With the advent of cheap computers able to handle the powerful thermometric titration software, development has now reached the stage where easy to use automated thermometric titration systems can in many cases offer a superior alternative to potentiometric titrimetry.
Potentiometric titration is a technique similar to direct titration of a redox reaction. It is a useful means of characterizing an acid. No indicator is used; instead the potential is measured across the analyte, typically an electrolyte solution. To do this, two electrodes are used, an indicator electrode and a reference electrode. Reference electrodes generally used are hydrogen electrodes, calomel electrodes, and silver chloride electrodes. The indicator electrode forms an electrochemical half cell with the interested ions in the test solution. The reference electrode forms the other half cell.
In chemistry, the equivalent concentration or normality of a solution is defined as the molar concentration ci divided by an equivalence factor feq:
Titration is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of a known reactant.
Conductometry is a measurement of electrolytic conductivity to monitor a progress of chemical reaction. Conductometry has notable application in analytical chemistry, where conductometric titration is a standard technique. In usual analytical chemistry practice, the term conductometry is used as a synonym of conductometric titration while the term conductimetry is used to describe non-titrative applications. Conductometry is often applied to determine the total conductance of a solution or to analyze the end point of titrations that include ions.
Total Base Number (TBN) is a measurement of basicity that is expressed in terms of the number of milligrams of potassium hydroxide per gram of oil sample. TBN is an important measurement in petroleum products, and the value varies depending on its application. TBN generally ranges from 6–8 mg KOH/g in modern lubricants, 7–10 mg KOH/g for general internal combustion engine use and 10–15 mg KOH/g for diesel engine operations. TBN is typically higher for marine grade lubricants, approximately 15-80 mg KOH/g, as the higher TBN values are designed to increase the operating period under harsh operating conditions, before the lubricant requires replacement.
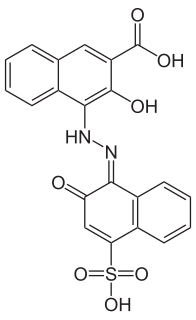
Calconcarboxylic acid is an azo dye that is used as an indicator for complexometric titrations of calcium with ethylenediaminetetraacetic acid (EDTA) in the presence of magnesium. Structurally, it is similar to eriochrome blue black R, which is obtained from calconcarboxylic acid by decarboxylation and reaction with sodium hydroxide.







