
The Montreal Protocol on Substances that Deplete the Ozone Layer, also known simply as the Montreal Protocol, is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. Open for signature on 16 September 1987, it was made pursuant to the 1985 Vienna Convention for the Protection of the Ozone Layer, which established the framework for international cooperation in addressing ozone depletion. The Montreal Protocol entered into force on 1 January 1989, and has since undergone nine revisions, in 1990 (London), 1991 (Nairobi), 1992 (Copenhagen), 1993 (Bangkok), 1995 (Vienna), 1997 (Montreal), 1998 (Australia), 1999 (Beijing) and 2016 (Kigali).

The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth's atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 15 to 35 kilometers (9 to 22 mi) above Earth, although its thickness varies seasonally and geographically.

Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone around Earth's polar regions. The latter phenomenon is referred to as the ozone hole. There are also springtime polar tropospheric ozone depletion events in addition to these stratospheric events.

The stratosphere is the second major layer of Earth's atmosphere, just above the troposphere, and below the mesosphere. The stratosphere is stratified (layered) in temperature, with warmer layers higher and cooler layers closer to the Earth; this increase of temperature with altitude is a result of the absorption of the Sun's ultraviolet radiation by the ozone layer. This is in contrast to the troposphere, near the Earth's surface, where temperature decreases with altitude. The border between the troposphere and stratosphere, the tropopause, marks where this temperature inversion begins. Near the equator, the lower edge of the stratosphere is as high as 20 km, at midlatitudes around 10 km, and at the poles about 7 km Temperatures range from an average of −51 °C near the tropopause to an average of −15 °C near the mesosphere. Stratospheric temperatures also vary within the stratosphere as the seasons change, reaching particularly low temperatures in the polar night (winter). Winds in the stratosphere can far exceed those in the troposphere, reaching near 60 m/s in the Southern polar vortex.
Chlorofluorocarbons (CFC’s) and hydrochlorofluorocarbons (HCFC’s) are fully or partly halogenated paraffin hydrocarbons that contain only carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivative of methane, ethane, and propane. They are also commonly known by the DuPont brand name Freon.

Ozone (O3) is a trace gas of the troposphere, with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer exists which is located between 10 and 50 kilometers above the earths surface. The troposphere is the lowest layer of the Earth's atmosphere. It extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground level or tropospheric ozone is created by chemical reactions between oxides of nitrogen (NOx gases) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, with a maximum concentration at the tropopause. About 90% of total ozone in the atmosphere is in the stratosphere, and 10% is in the troposphere. Although tropospheric ozone is less concentrated than stratospheric ozone, it is of concern because of its health effects. Ozone in the troposphere is considered a greenhouse gas, and may contribute to global warming.

Mario José Molina-Pasquel Henríquez, known as Mario Molina, was a Mexican chemist. He played a pivotal role in the discovery of the Antarctic ozone hole, and was a co-recipient of the 1995 Nobel Prize in Chemistry for his role in discovering the threat to the Earth's ozone layer from chlorofluorocarbon (CFC) gases. He was the first Mexican-born scientist to receive a Nobel Prize in Chemistry and the third Mexican born person to receive the Nobel award.

The ozone–oxygen cycle is the process by which ozone is continually regenerated in Earth's stratosphere, converting ultraviolet radiation (UV) into heat. In 1930 Sydney Chapman resolved the chemistry involved. The process is commonly called the Chapman cycle by atmospheric scientists.

Frank Sherwood "Sherry" Rowland was an American Nobel laureate and a professor of chemistry at the University of California, Irvine. His research was on atmospheric chemistry and chemical kinetics. His best-known work was the discovery that chlorofluorocarbons contribute to ozone depletion.

Polar stratospheric clouds (PSCs) are clouds in the winter polar stratosphere at altitudes of 15,000–25,000 m (49,000–82,000 ft). They are best observed during civil twilight, when the Sun is between 1 and 6 degrees below the horizon, as well as in winter and in more northerly latitudes. One main type of PSC is made up mostly of supercooled droplets of water and nitric acid and is implicated in the formation of ozone holes. The other main type consists only of ice crystals which are not harmful. This type of PSC is also referred to as nacreous.
The ozone depletion potential (ODP) of a chemical compound is the relative amount of degradation to the ozone layer it can cause, with trichlorofluoromethane being fixed at an ODP of 1.0. Chlorodifluoromethane (R-22), for example, has an ODP of 0.05. CFC 11, or R-11 has the maximum potential amongst chlorocarbons because of the presence of three chlorine atoms in the molecule.
Trichlorofluoromethane, also called freon-11, CFC-11, or R-11, is a chlorofluorocarbon (CFC). It is a colorless, faintly ethereal, and sweetish-smelling liquid that boils around room temperature. CFC-11 is a Class 1 ozone-depleting substance which damages Earth's protective stratospheric ozone layer.
Chlorine monoxide is a chemical radical with the chemical formula . It plays an important role in the process of ozone depletion. In the stratosphere, chlorine atoms react with ozone molecules to form chlorine monoxide and oxygen.
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane or CFC-113, is a chlorofluorocarbon. It has the formula Cl2FC-CClF2. This colorless, volatile liquid is a versatile solvent.

SAGE III on ISS is the fourth generation of a series of NASA Earth-observing instruments, known as the Stratospheric Aerosol and Gas Experiment. The first SAGE III instrument was launched on the Russian Meteor (satellite) spacecraft. The recently revised SAGE III will be mounted to the International Space Station where it will use the unique vantage point of ISS to make long-term measurements of ozone, aerosols, water vapor, and other gases in Earth's atmosphere.

Bromofluorocarbons (BFCs) are molecules based on carbon, bromine, and fluorine. The most common use has traditionally been in fire suppression systems. The brand name "Halon" is frequently used interchangeably for BFCs. However, not all Halons are technically BFCs.
Ozone depletion and climate change, or Ozone hole and global warming in more popular terms, are environmental challenges whose connections have been explored and which have been compared and contrasted, for example in terms of global regulation, in various studies and books.
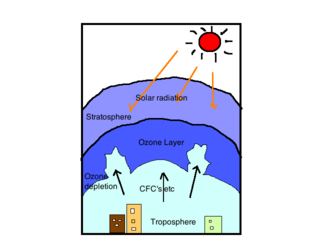
Very short-lived substances (VSLS) are ozone-depleting halogen-containing substances found in the stratosphere. These substances have very short lifetimes, typically less than 6 months. VSLS are responsible for atmospheric damage once they enter the stratosphere and are a contributing factor to the destruction of ozone.
Azadeh Tabazadeh is an Iranian geophysicist and author known for her work in atmospheric science, work which has improved our understanding of the reactions that affect ozone depletion and highlighted the impact human activity has on the atmosphere.
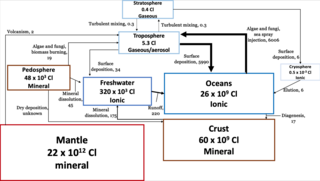
The chlorine cycle (Cl) is the biogeochemical cycling of chlorine through the atmosphere, hydrosphere, biosphere, and lithosphere. Chlorine is most commonly found as inorganic chloride ions, or a number of chlorinated organic forms. Over 5,000 biologically-produced chlorinated organics have been identified.











