Melamine is an organic compound with the formula C3H6N6. This white solid is a trimer of cyanamide, with a 1,3,5-triazine skeleton. Like cyanamide, it contains 66% nitrogen by mass, and its derivatives have fire-retardant properties due to its release of nitrogen gas when burned or charred. Melamine can be combined with formaldehyde and other agents to produce melamine resins. Such resins are characteristically durable thermosetting plastic used in high pressure decorative laminates such as Formica, melamine dinnerware including cooking utensils, plates, plastic products, laminate flooring, and dry erase boards. Melamine foam is used as insulation, soundproofing material and in polymeric cleaning products, such as Magic Eraser.

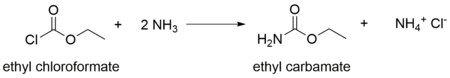
In organic chemistry, a carbamate is a category of organic compounds with the general formula R2NC(O)OR and structure >N−C(=O)−O−, which are formally derived from carbamic acid. The term includes organic compounds, formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion H2NCOO−.

Gas chromatography–mass spectrometry (GC–MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC–MS include drug detection, fire investigation, environmental analysis, explosives investigation, food and flavor analysis, and identification of unknown samples, including that of material samples obtained from planet Mars during probe missions as early as the 1970s. GC–MS can also be used in airport security to detect substances in luggage or on human beings. Additionally, it can identify trace elements in materials that were previously thought to have disintegrated beyond identification. Like liquid chromatography–mass spectrometry, it allows analysis and detection even of tiny amounts of a substance.

Liquor or distilled beverage is an alcoholic drink produced by the distillation of grains, fruits, vegetables, or sugar that have already gone through alcoholic fermentation. Other terms for liquor include spirit, spirituous liquor or hard liquor. While the word liquor ordinarily refers to distilled alcoholic spirits rather than beverages produced by fermentation alone, it can sometimes be used more broadly to refer to any alcoholic beverage.

Ethylbenzene is an organic compound with the formula C6H5CH2CH3. It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as a reaction intermediate in the production of styrene, the precursor to polystyrene, a common plastic material. In 2012, more than 99% of ethylbenzene produced was consumed in the production of styrene.
IARC group 2A agents are substances and exposure circumstances that have been classified as probable carcinogens by the International Agency for Research on Cancer (IARC). This designation is applied when there is limited evidence of carcinogenicity in humans, as well as sufficient evidence of carcinogenicity in experimental animals. In some cases, an agent may be classified in this group when there is inadequate evidence of carcinogenicity in humans along with sufficient evidence of carcinogenicity in experimental animals and strong evidence that the carcinogenesis is mediated by a mechanism that also operates in humans. Exceptionally, an agent may be classified in this group solely on the basis of limited evidence of carcinogenicity in humans.

Theanine, commonly known as L-theanine and sometimes L-gamma-glutamylethylamide or N5-ethyl-L-glutamine, is an amino acid analogue of the proteinogenic amino acids L-glutamate and L-glutamine and is found primarily in particular plant and fungal species. It was discovered as a constituent of green tea in 1949 and isolated from gyokuro leaves in 1950, thus rendering it a natural product. It constitutes about 1–2% of the dry weight of green tea leaves.

N-Nitrosonornicotine (NNN) is a tobacco-specific nitrosamine produced during the curing and processing of tobacco.

Caffeic acid is an organic compound with the formula (HO)2C6H3CH=CHCO2H. It is a polyphenol. It is a yellow solid. Structurally, it is classified as a hydroxycinnamic acid. The molecule consists of both phenolic and acrylic functional groups. It is found in all plants as an intermediate in the biosynthesis of lignin, one of the principal components of biomass and its residues. It is unrelated to caffeine.

Dimethyl dicarbonate (DMDC) is a colorless liquid with a pungent odor at high concentration at room temperature. It is primarily used as a beverage preservative, processing aid, or sterilant being highly active against typical beverage spoiling microorganisms like yeast, bacteria, or mould.

Methyl carbamate (also called methylurethane, or urethylane) is an organic compound and the simplest ester of carbamic acid (H2NCO2H). It is a colourless solid.

In food processing, fermentation is the conversion of carbohydrates to alcohol or organic acids using microorganisms—yeasts or bacteria—without an oxidizing agent being used in the reaction. Fermentation usually implies that the action of microorganisms is desired. The science of fermentation is known as zymology or zymurgy.

In molecular genetics, a DNA adduct is a segment of DNA bound to a cancer-causing chemical. This process could lead to the development of cancerous cells, or carcinogenesis. DNA adducts in scientific experiments are used as biomarkers of exposure. They are especially useful in quantifying an organism's exposure to a carcinogen. The presence of such an adduct indicates prior exposure to a potential carcinogen, but it does not necessarily indicate the presence of cancer in the subject animal.

tert-Amyl alcohol (TAA) or 2-methylbutan-2-ol (2M2B), is a branched pentanol.

The relationship between alcohol and breast cancer is clear: drinking alcoholic beverages, including wine, beer, or liquor, is a risk factor for breast cancer, as well as some other forms of cancer. Drinking alcohol causes more than 100,000 cases of breast cancer worldwide every year. Globally, almost one in 10 cases of breast cancer is caused by women drinking alcoholic beverages. Drinking alcoholic beverages is among the most common modifiable risk factors.

Ptaquiloside is a norsesquiterpene glucoside produced by bracken ferns during metabolism. It is identified to be the main carcinogen of the ferns and to be responsible for their biological effects, such as haemorrhagic disease and bright blindness in livestock and oesophageal, gastric cancer in humans. Ptaquiloside has an unstable chemical structure and acts as a DNA alkylating agent under physiological conditions. It was first isolated and characterized by Yamada and co-workers in 1983.
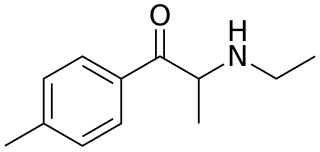
4-Methylethcathinone or 4-MEC is a chemical that bears a chemical resemblance to mephedrone. Due to its similarity to mephedrone, it is thought to be a stimulant and entactogen drug of the phenethylamine, amphetamine, and cathinone chemical classes. It has been marketed alone or in mixtures with other substituted cathinones under the name "NRG-2", although other blends such as "NRG-1" may have been more ambiguous with their ingredients.
The Swedish ethyl acetate method (SweEt) is a method for chemical analysis of pesticide residues in food using ethyl acetate as an extraction medium followed by analysis with liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-tandem mass spectrometry (GC-MS/MS). It was developed by the Swedish National Food Agency for quantitative analysis of over 500 pesticides in fruits, vegetables, cereals and products of animal origin.

Animocarb (Matacil) is an organic chemical compound with the molecular formula C11H16N2O2. It has a colorless or white crystal-like appearance and is most commonly used as an insecticide.

Dimethylcarbamoyl chloride (DMCC) is a reagent for transferring a dimethylcarbamoyl group to alcoholic or phenolic hydroxyl groups forming dimethyl carbamates, usually having pharmacological or pesticidal activities. Because of its high toxicity and its carcinogenic properties shown in animal experiments and presumably also in humans, dimethylcarbamoyl chloride can only be used under stringent safety precautions.