
Food coloring, or color additive, is any dye, pigment, or substance that imparts color when it is added to food or drink. They be supplied as liquids, powders, gels, or pastes. Food coloring is used in both commercial food production and domestic cooking. Food colorants are also used in a variety of non-food applications, including cosmetics, pharmaceuticals, home craft projects, and medical devices. Colorings may be natural or artificial/synthetic.
A biocide is defined in the European legislation as a chemical substance or microorganism intended to destroy, deter, render harmless, or exert a controlling effect on any harmful organism. The US Environmental Protection Agency (EPA) uses a slightly different definition for biocides as "a diverse group of poisonous substances including preservatives, insecticides, disinfectants, and pesticides used for the control of organisms that are harmful to human or animal health or that cause damage to natural or manufactured products". When compared, the two definitions roughly imply the same, although the US EPA definition includes plant protection products and some veterinary medicines.

A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is a document that lists information relating to occupational safety and health for the use of various substances and products. SDSs are a widely used system for cataloguing information on chemicals, chemical compounds, and chemical mixtures. SDS information may include instructions for the safe use and potential hazards associated with a particular material or product, along with spill-handling procedures. The older MSDS formats could vary from source to source within a country depending on national requirements; however, the newer SDS format is internationally standardized.

Sunset yellow FCF is a petroleum-derived orange azo dye with a pH dependent maximum absorption at about 480 nm at pH 1 and 443 nm at pH 13 with a shoulder at 500 nm. When added to foods sold in the United States it is known as FD&C Yellow 6; when sold in Europe, it is denoted by E Number E110.

Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) is a European Union regulation dating from 18 December 2006. REACH addresses the production and use of chemical substances, and their potential impacts on both human health and the environment. Its 849 pages took seven years to pass, and it has been described as the most complex legislation in the Union's history and the most important in 20 years. It is the strictest law to date regulating chemical substances and will affect industries throughout the world. REACH entered into force on 1 June 2007, with a phased implementation over the next decade. The regulation also established the European Chemicals Agency, which manages the technical, scientific and administrative aspects of REACH.

Waste hierarchy is a tool used in the evaluation of processes that protect the environment alongside resource and energy consumption from most favourable to least favourable actions. The hierarchy establishes preferred program priorities based on sustainability. To be sustainable, waste management cannot be solved only with technical end-of-pipe solutions and an integrated approach is necessary.

The Dangerous Substances Directive was one of the main European Union laws concerning chemical safety, until its full replacement by the new regulation CLP Regulation (2008), starting in 2016. It was made under Article 100 of the Treaty of Rome. By agreement, it is also applicable in the EEA, and compliance with the directive will ensure compliance with the relevant Swiss laws. The Directive ceased to be in force on 31 May 2015 and was repealed by Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006.
In the experimental (non-clinical) research arena, good laboratory practice or GLP is a quality system of management controls for research laboratories and organizations to ensure the uniformity, consistency, reliability, reproducibility, quality, and integrity of products in development for human or animal health through non-clinical safety tests; from physio-chemical properties through acute to chronic toxicity tests.

The European Chemicals Agency is an agency of the European Union which manages the technical and administrative aspects of the implementation of the European Union regulation called Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). ECHA is the driving force among regulatory authorities in implementing the EU's chemicals legislation. ECHA has to ascertain that companies comply with the legislation, advances the safe use of chemicals, provides information on chemicals and addresses chemicals of concern. It is located in Helsinki, Finland. ECHA is an independent and mature regulatory agency established by REACH. It is not a subsidiary entity of the European Commission.

Alternatives to animal testing are the development and implementation of test methods that avoid the use of live animals.
IUCLID is a software application to capture, store, maintain and exchange data on intrinsic and hazard properties of chemical substances. Distributed free of charge, the software is especially useful to chemical industry companies and to government authorities. It is the key tool for chemical industry to fulfill data submission obligations under REACH, the most important European Union legal document covering the production and use of chemical substances. The software is maintained by the European Chemicals Agency, ECHA. The latest version, version 6, was made available on 29 April 2016.
The European Chemicals Bureau (ECB) was the focal point for the data and assessment procedure on dangerous chemicals within the European Union (EU). The ECB was located in Ispra, Italy, within the Joint Research Centre (JRC) of the European Commission. In 2008 the ECB completed its mandate. Some of its activities were taken over by the European Chemicals Agency (ECHA); others remained within the Joint Research Centre. The history of the ECB has been published as a JRC technical report.

The Medical Device Directive — Council Directive 93/42/EEC of 14 June 1993 concerning medical devices — is intended to harmonise the laws relating to medical devices within the European Union. The MD Directive is a 'New Approach' Directive and consequently in order for a manufacturer to legally place a medical device on the European market the requirements of the MD Directive have to be met. Manufacturers' products meeting 'harmonised standards' have a presumption of conformity to the Directive. Products conforming with the MD Directive must have a CE mark applied. The Directive was most recently reviewed and amended by the 2007/47/EC and a number of changes were made. Compliance with the revised directive became mandatory on 21 March 2010.

The CLP Regulation is a European Union regulation from 2008, which aligns the European Union system of classification, labelling and packaging of chemical substances and mixtures to the Globally Harmonised System (GHS). It is expected to facilitate global trade and the harmonised communication of hazard information of chemicals and to promote regulatory efficiency. It complements the 2006 Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Regulation and replaces an older system contained in the Dangerous Substances Directive (67/548/EEC) and the Dangerous Preparations Directive (1999/45/EC).

Council Directive 76/768/EEC of 27 July 1976 on the approximation of the laws of the Member States relating to cosmetic products was the main European Union law on the safety of cosmetics. It was made under Art. 100 of the Treaty of Rome. By agreement, it was also applicable in the European Economic Area.
The Substitute It Now! List is a database developed by the International Chemical Secretariat (ChemSec) of chemicals the uses of which are likely to become legally restricted under EU REACH regulation. The list is being used by public interest groups as a campaign tool to advocate for increasing the pace of implementation of REACH and by commercial interests to identify substances for control in chemicals management programmes.
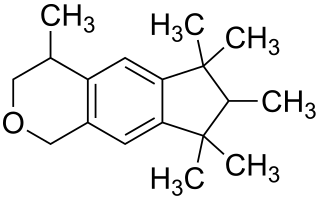
Galaxolide is a synthetic musk with a clean sweet musky floral woody odor used in fragrances. It is one of the musk components that perfume and cologne manufacturers use to add a musk odor to their products. Galaxolide was first synthesized in 1965, and used in the late 1960s in some fabric softeners and detergents. High concentrations were also incorporated in fine fragrances.

Directive 2010/63/EU is the European Union (EU) legislation "on the protection of animals used for scientific purposes" and is one of the most stringent ethical and welfare standards worldwide.
EC Regulation 1223/2009 on cosmetics sets binding requirements for cosmetic products that have been made available on the market within the European Union. Manufacturers of products that fall under the category or cosmetics are required to abide by this regulation as they prepare their initial release of products and while continuing to sell said products within the Member States of the EU.

A pesticide, also called Plant Protection Product (PPP), which is a term used in regulatory documents, consists of several different components. The active ingredient in a pesticide is called “active substance” and these active substances either consist of chemicals or micro-organisms. The aims of these active substances are to specifically take action against organisms that are harmful to plants. In other words, active substances are the active components against pests and plant diseases.









