
The ovary is a gonad in the female reproductive system that produces ova. When an ovum is released, this travels through the fallopian tube into the uterus. There is an ovary found on the left and the right side of the body. The ovaries also secrete hormones that play a role in the menstrual cycle and fertility. The ovary progresses through many stages beginning in the prenatal period through menopause. It is also an endocrine gland because of the various hormones that it secretes.

Oogenesis, ovogenesis, or oögenesis is the differentiation of the ovum into a cell competent to further develop when fertilized. It is developed from the primary oocyte by maturation. Oogenesis is initiated in the embryonic stage.

An ovarian follicle is a roughly spheroid cellular aggregation set found in the ovaries. It secretes hormones that influence stages of the menstrual cycle. At the time of puberty, women have approximately 200,000 to 300,000 follicles, each with the potential to release an egg cell (ovum) at ovulation for fertilization. These eggs are developed once every menstrual cycle with around 450–500 being ovulated during a woman's reproductive lifetime.

A granulosa cell or follicular cell is a somatic cell of the sex cord that is closely associated with the developing female gamete in the ovary of mammals.

In biology, folliculogenesis is the maturation of the ovarian follicle, a densely packed shell of somatic cells that contains an immature oocyte. Folliculogenesis describes the progression of a number of small primordial follicles into large preovulatory follicles that occurs in part during the menstrual cycle.

Ovarian reserve is a term that is used to determine the capacity of the ovary to provide egg cells that are capable of fertilization resulting in a healthy and successful pregnancy. With advanced maternal age the number of egg cell that can be successfully recruited for a possible pregnancy declines, constituting a major factor in the inverse correlation between age and female fertility.

Bone morphogenetic protein 15 (BMP-15) is a protein that in humans is encoded by the BMP15 gene. It is involved in folliculogenesis, the process in which primordial follicles develop into pre-ovulatory follicles.
Controlled ovarian hyperstimulation is a technique used in assisted reproduction involving the use of fertility medications to induce ovulation by multiple ovarian follicles. These multiple follicles can be taken out by oocyte retrieval for use in in vitro fertilisation (IVF), or be given time to ovulate, resulting in superovulation which is the ovulation of a larger-than-normal number of eggs, generally in the sense of at least two. When ovulated follicles are fertilised in vivo, whether by natural or artificial insemination, there is a very high risk of a multiple pregnancy.

In vitro maturation (IVM) is the technique of letting the contents of ovarian follicles and the oocytes inside mature in vitro. It can be offered to women with infertility problems, combined with In Vitro Fertilization (IVF), offering women pregnancy without ovarian stimulation.
Poor ovarian reserve is a condition of low fertility characterized by 1): low numbers of remaining oocytes in the ovaries or 2) possibly impaired preantral oocyte development or recruitment. Recent research suggests that premature ovarian aging and premature ovarian failure may represent a continuum of premature ovarian senescence. It is usually accompanied by high FSH levels.
Transvaginal oocyte retrieval (TVOR), also referred to as oocyte retrieval (OCR), is a technique used in in vitro fertilization (IVF) in order to remove oocytes from the ovary of a woman, enabling fertilization outside the body. Transvaginal oocyte retrieval is more properly referred to as transvaginal ovum retrieval when the oocytes have matured into ova, as is normally the case in IVF. It can be also performed for egg donation, oocyte cryopreservation and other assisted reproduction technology such as ICSI.
Fertility preservation is the effort to help cancer patients retain their fertility, or ability to procreate. Research into how cancer, ageing and other health conditions effect reproductive health and preservation options are growing. Specifically sparked in part by the increase in the survival rate of cancer patients.
Ovarian tissue cryopreservation is cryopreservation of tissue of the ovary of a female.
Oogonial stem cells (OSCs), also known as egg precursor cells or female germline cells, are diploid germline cells with stem cell characteristics: the ability to renew and differentiate into other cell types, different from their tissue of origin. Present in invertebrates and some lower vertebrate species, they have been extensively studied in Caenorhabditis elegans, Drosophila melanogaster. OSCs allow the production of new female reproductive cells (oocytes) by the process of oogenesis during an organism's reproductive life.

Roger Gordon Gosden is a British-American physiologist in the field of female reproductive medicine. His scientific research focused on understanding the basic biology of development and senescence of ovaries in women, including mathematically modeling those processes. He did important translational research on ovarian tissue cryopreservation and transplantation.
Ovarian follicle activation can be defined as primordial follicles in the ovary moving from a quiescent (inactive) to a growing phase. The primordial follicle in the ovary is what makes up the “pool” of follicles that will be induced to enter growth and developmental changes that change them into pre-ovulatory follicles, ready to be released during ovulation. The process of development from a primordial follicle to a pre-ovulatory follicle is called folliculogenesis.
Ovarian follicle dominance is the process where one or more follicles are selected per cycle to ovulate.

An artificial ovary is a potential fertility preservation treatment that aims to mimic the function of the natural ovary.
Ovarian culture is an in-vitro process that allows for the investigation of the development, toxicology and pathology of the ovary. This technique can also be used to study possible applications of fertility treatments e.g. isolating oocytes from primordial ovarian follicles that could be used for fertilisation.
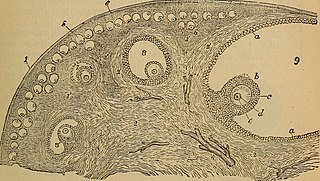
Ovarian stem cells are oocytes formed in ovarian follicle before birth in female mammals. They do not form post-natally, and are depleted throughout reproductive life. In humans it is estimated that 500,000–1,000,000 primordial follicles are present at birth, decreasing rapidly with age until roughly age 51 when ovulation stops, resulting in menopause. The origin of these oocytes remains under discussion. The publication of a study in 2004 proposing germ cell renewal in adult mice sparked a debate on the possibility of stem cells in the postnatal ovary. An increasing number of studies suggest that stem cells exist within the mammalian ovary and can be manipulated in vitro to produce oocytes, but whether such ovarian stem cells have the potential to differentiate into oocytes remains uncertain.











