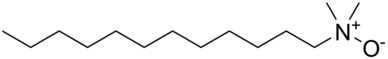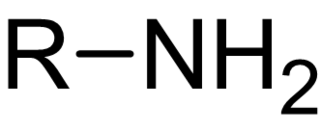
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups. Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, the human impact in recent years could have an effect on the biological communities that depend on it.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word "surfactant" is a blend of surface-active agent, coined c. 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt.

Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed industrially to produce ethanol, isopropanol, and butan-2-ol.
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although the name ammonium hydroxide suggests a salt with the composition [NH+
4][OH−
], it is actually impossible to isolate samples of NH4OH. The ions NH+
4 and OH− do not account for a significant fraction of the total amount of ammonia except in extremely dilute solutions.
Hydrobromic acid is an aqueous solution of hydrogen bromide. It is a strong acid formed by dissolving the diatomic molecule hydrogen bromide (HBr) in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at 124.3 °C (255.7 °F) and contains 47.6% HBr by mass, which is 8.77 mol/L. Hydrobromic acid is one of the strongest mineral acids known.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.

In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure [NR4]+, where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.

In chemistry, the term phosphonium describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.

A hydrohalogenation reaction is the electrophilic addition of hydrogen halides like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes.
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base.

Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with cyanide in the presence of ammonia. The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid. The method is used for the commercial production of racemic methionine from methional.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.
Amine alkylation (amino-dehalogenation) is a type of organic reaction between an alkyl halide and ammonia or an amine. The reaction is called nucleophilic aliphatic substitution, and the reaction product is a higher substituted amine. The method is widely used in the laboratory, but less so industrially, where alcohols are often preferred alkylating agents.
In nitrile reduction a nitrile is reduced to either an amine or an aldehyde with a suitable chemical reagent.
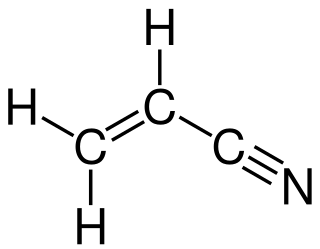
In organic chemistry, ammoxidation is a process for the production of nitriles using ammonia and oxygen. It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio. The usual substrates are alkenes. Several million tons of acrylonitrile are produced in this way annually:
The Ritter reaction is a chemical reaction that transforms a nitrile into an N-alkyl amide using various electrophilic alkylating reagents. The original reaction formed the alkylating agent using an alkene in the presence of a strong acid.

Tetraethylammonium chloride (TEAC) is a quaternary ammonium compound with the chemical formula [N(CH2CH3)4]+Cl−, sometimes written as [NEt4]Cl. In appearance, it is a hygroscopic, colorless, crystalline solid. It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis.