Systems biology is the computational and mathematical analysis and modeling of complex biological systems. It is a biology-based interdisciplinary field of study that focuses on complex interactions within biological systems, using a holistic approach to biological research.

Metabolomics is the scientific study of chemical processes involving metabolites, the small molecule substrates, intermediates, and products of cell metabolism. Specifically, metabolomics is the "systematic study of the unique chemical fingerprints that specific cellular processes leave behind", the study of their small-molecule metabolite profiles. The metabolome represents the complete set of metabolites in a biological cell, tissue, organ, or organism, which are the end products of cellular processes. Messenger RNA (mRNA), gene expression data, and proteomic analyses reveal the set of gene products being produced in the cell, data that represents one aspect of cellular function. Conversely, metabolic profiling can give an instantaneous snapshot of the physiology of that cell, and thus, metabolomics provides a direct "functional readout of the physiological state" of an organism. There are indeed quantifiable correlations between the metabolome and the other cellular ensembles, which can be used to predict metabolite abundances in biological samples from, for example mRNA abundances. One of the ultimate challenges of systems biology is to integrate metabolomics with all other -omics information to provide a better understanding of cellular biology.

The metabolome refers to the complete set of small-molecule chemicals found within a biological sample. The biological sample can be a cell, a cellular organelle, an organ, a tissue, a tissue extract, a biofluid or an entire organism. The small molecule chemicals found in a given metabolome may include both endogenous metabolites that are naturally produced by an organism as well as exogenous chemicals that are not naturally produced by an organism.
Modelling biological systems is a significant task of systems biology and mathematical biology. Computational systems biology aims to develop and use efficient algorithms, data structures, visualization and communication tools with the goal of computer modelling of biological systems. It involves the use of computer simulations of biological systems, including cellular subsystems, to both analyze and visualize the complex connections of these cellular processes.

Metabolic engineering is the practice of optimizing genetic and regulatory processes within cells to increase the cell's production of a certain substance. These processes are chemical networks that use a series of biochemical reactions and enzymes that allow cells to convert raw materials into molecules necessary for the cell's survival. Metabolic engineering specifically seeks to mathematically model these networks, calculate a yield of useful products, and pin point parts of the network that constrain the production of these products. Genetic engineering techniques can then be used to modify the network in order to relieve these constraints. Once again this modified network can be modeled to calculate the new product yield.
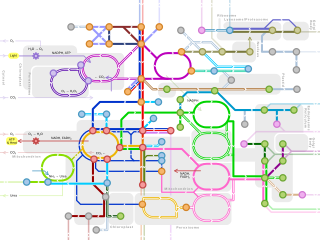
A metabolic network is the complete set of metabolic and physical processes that determine the physiological and biochemical properties of a cell. As such, these networks comprise the chemical reactions of metabolism, the metabolic pathways, as well as the regulatory interactions that guide these reactions.
Metabolic network modelling, also known as metabolic network reconstruction or metabolic pathway analysis, allows for an in-depth insight into the molecular mechanisms of a particular organism. In particular, these models correlate the genome with molecular physiology. A reconstruction breaks down metabolic pathways into their respective reactions and enzymes, and analyzes them within the perspective of the entire network. In simplified terms, a reconstruction collects all of the relevant metabolic information of an organism and compiles it in a mathematical model. Validation and analysis of reconstructions can allow identification of key features of metabolism such as growth yield, resource distribution, network robustness, and gene essentiality. This knowledge can then be applied to create novel biotechnology.

KEGG is a collection of databases dealing with genomes, biological pathways, diseases, drugs, and chemical substances. KEGG is utilized for bioinformatics research and education, including data analysis in genomics, metagenomics, metabolomics and other omics studies, modeling and simulation in systems biology, and translational research in drug development.
Phenomics is the systematic study of traits that make up a phenotype. It was coined by UC Berkeley and LBNL scientist Steven A. Garan. As such, it is a transdisciplinary area of research that involves biology, data sciences, engineering and other fields. Phenomics is concerned with the measurement of the phenotype where a phenome is a set of traits that can be produced by a given organism over the course of development and in response to genetic mutation and environmental influences. It is also important to remember that an organisms phenotype changes with time. The relationship between phenotype and genotype enables researchers to understand and study pleiotropy. Phenomics concepts are used in functional genomics, pharmaceutical research, metabolic engineering, agricultural research, and increasingly in phylogenetics.
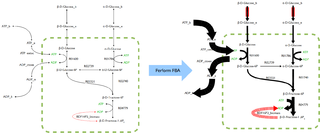
Flux balance analysis (FBA) is a mathematical method for simulating metabolism in genome-scale reconstructions of metabolic networks. In comparison to traditional methods of modeling, FBA is less intensive in terms of the input data required for constructing the model. Simulations performed using FBA are computationally inexpensive and can calculate steady-state metabolic fluxes for large models in a few seconds on modern personal computers. The related method of metabolic pathway analysis seeks to find and list all possible pathways between metabolites.
The Systems Biology Markup Language (SBML) is a representation format, based on XML, for communicating and storing computational models of biological processes. It is a free and open standard with widespread software support and a community of users and developers. SBML can represent many different classes of biological phenomena, including metabolic networks, cell signaling pathways, regulatory networks, infectious diseases, and many others. It has been proposed as a standard for representing computational models in systems biology today.
Flux, or metabolic flux is the rate of turnover of molecules through a metabolic pathway. Flux is regulated by the enzymes involved in a pathway. Within cells, regulation of flux is vital for all metabolic pathways to regulate the pathway's activity under different conditions. Flux is therefore of great interest in metabolic network modelling, where it is analysed via flux balance analysis and metabolic control analysis.

Metabolic flux analysis (MFA) is an experimental fluxomics technique used to examine production and consumption rates of metabolites in a biological system. At an intracellular level, it allows for the quantification of metabolic fluxes, thereby elucidating the central metabolism of the cell. Various methods of MFA, including isotopically stationary metabolic flux analysis, isotopically non-stationary metabolic flux analysis, and thermodynamics-based metabolic flux analysis, can be coupled with stoichiometric models of metabolism and mass spectrometry methods with isotopic mass resolution to elucidate the transfer of moieties containing isotopic tracers from one metabolite into another and derive information about the metabolic network. Metabolic flux analysis (MFA) has many applications such as determining the limits on the ability of a biological system to produce a biochemical such as ethanol, predicting the response to gene knockout, and guiding the identification of bottleneck enzymes in metabolic networks for metabolic engineering efforts.
Biochemical systems theory is a mathematical modelling framework for biochemical systems, based on ordinary differential equations (ODE), in which biochemical processes are represented using power-law expansions in the variables of the system.

Reinhart Heinrich was a German biophysicist.
Rhodobacter sphaeroides is a kind of purple bacterium; a group of bacteria that can obtain energy through photosynthesis. Its best growth conditions are anaerobic phototrophy and aerobic chemoheterotrophy in the absence of light. R. sphaeroides is also able to fix nitrogen. It is remarkably metabolically diverse, as it is able to grow heterotrophically via fermentation and aerobic and anaerobic respiration. Such a metabolic versatility has motivated the investigation of R. sphaeroides as a microbial cell factory for biotechnological applications.
Bernhard Ørn Palsson is the Galletti Professor of Bioengineering and an adjunct professor of Medicine at the University of California, San Diego.

Stefan Schuster is a German biophysicist. He is professor for bioinformatics at the University of Jena.
David A. Fell is a British biochemist and professor of systems biology at Oxford Brookes University. He has published over 200 publications, including a textbook on Understanding the control of metabolism in 1996.

Markus Ralser is an Italian biologist. His main research interest is metabolism of microorganisms. He is also known for his work on the origin of metabolism during the origin of life, and proteomics.











