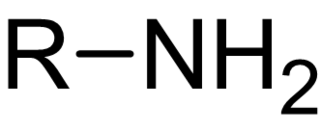
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.

Diatomic molecules are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen or oxygen, then it is said to be homonuclear. Otherwise, if a diatomic molecule consists of two different atoms, such as carbon monoxide or nitric oxide, the molecule is said to be heteronuclear. The bond in a homonuclear diatomic molecule is non-polar.
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2. Thus, sulfur monoxide and disulfur dioxide, both compounds of sulfur and oxygen, have the same empirical formula. However, their molecular formulas, which express the number of atoms in each molecule of a chemical compound, are not the same.

A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.

In chemistry, the molar mass of a chemical compound is defined as the ratio between the mass and the amount of substance of any sample of said compound. The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities.

The structural formula of a chemical compound is a graphic representation of the molecular structure, showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Unlike other chemical formula types, which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a more complete geometric representation of the molecular structure. For example, many chemical compounds exist in different isomeric forms, which have different enantiomeric structures but the same molecular formula. There are multiple types of ways to draw these structural formulas such as: Lewis Structures, condensed formulas, skeletal formulas, Newman projections, Cyclohexane conformations, Haworth projections, and Fischer projections.
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities are on the right-hand side with a plus sign between the entities in both the reactants and the products, and an arrow that points towards the products to show the direction of the reaction. The chemical formulas may be symbolic, structural, or intermixed. The coefficients next to the symbols and formulas of entities are the absolute values of the stoichiometric numbers. The first chemical equation was diagrammed by Jean Beguin in 1615.
In atomic physics, a partial charge is a non-integer charge value when measured in elementary charge units. It is represented by the Greek lowercase delta (𝛿), namely 𝛿− or 𝛿+.
In mathematics, computer science, and logic, rewriting covers a wide range of methods of replacing subterms of a formula with other terms. Such methods may be achieved by rewriting systems. In their most basic form, they consist of a set of objects, plus relations on how to transform those objects.
In theoretical computer science and mathematical logic a string rewriting system (SRS), historically called a semi-Thue system, is a rewriting system over strings from a alphabet. Given a binary relation between fixed strings over the alphabet, called rewrite rules, denoted by , an SRS extends the rewriting relation to all strings in which the left- and right-hand side of the rules appear as substrings, that is , where , , , and are strings.

A bond graph is a graphical representation of a physical dynamic system. It allows the conversion of the system into a state-space representation. It is similar to a block diagram or signal-flow graph, with the major difference that the arcs in bond graphs represent bi-directional exchange of physical energy, while those in block diagrams and signal-flow graphs represent uni-directional flow of information. Bond graphs are multi-energy domain and domain neutral. This means a bond graph can incorporate multiple domains seamlessly.
In mathematical logic, an atomic formula is a formula with no deeper propositional structure, that is, a formula that contains no logical connectives or equivalently a formula that has no strict subformulas. Atoms are thus the simplest well-formed formulas of the logic. Compound formulas are formed by combining the atomic formulas using the logical connectives.

In the analysis of the molecular formula of organic molecules, the degree of unsaturation (DU) (also known as the index of hydrogen deficiency (IHD), double bond equivalents (DBE), or unsaturation index) is a calculation that determines the total number of rings and π bonds. A formula is used in organic chemistry to help draw chemical structures. It does not give any information about those components individually—the specific number of rings, or of double bonds (one π bond each), or of triple bonds (two π bonds each). The final structure is verified with use of NMR, mass spectrometry and IR spectroscopy, as well as qualitative inspection. It is based on comparing the actual molecular formula to what would be a possible formula if the structure were saturated—having no rings and containing only σ bonds—with all atoms having their standard valence.
In mathematics, a symmetry operation is a geometric transformation of an object that leaves the object looking the same after it has been carried out. For example, a 1⁄3 turn rotation of a regular triangle about its center, a reflection of a square across its diagonal, a translation of the Euclidean plane, or a point reflection of a sphere through its center are all symmetry operations. Each symmetry operation is performed with respect to some symmetry element. Symmetry operations can be classified either as point symmetry operations or as travel symmetry operations.

The mass recorded by a mass spectrometer can refer to different physical quantities depending on the characteristics of the instrument and the manner in which the mass spectrum is displayed.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.

In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Diamond and graphite are a familiar example; they are isomers of carbon. Isomerism refers to the existence or possibility of isomers.
In physical chemistry, there are numerous quantities associated with chemical compounds and reactions; notably in terms of amounts of substance, activity or concentration of a substance, and the rate of reaction. This article uses SI units.




















