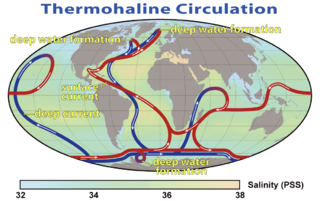
Oceanography, also known as oceanology and ocean science, is the scientific study of the oceans. It is an important Earth science, which covers a wide range of topics, including ecosystem dynamics; ocean currents, waves, and geophysical fluid dynamics; plate tectonics and the geology of the sea floor; and fluxes of various chemical substances and physical properties within the ocean and across its boundaries. These diverse topics reflect multiple disciplines that oceanographers utilize to glean further knowledge of the world ocean, including astronomy, biology, chemistry, climatology, geography, geology, hydrology, meteorology and physics. Paleoceanography studies the history of the oceans in the geologic past. An oceanographer is a person who studies many matters concerned with oceans, including marine geology, physics, chemistry and biology.

Ocean Thermal Energy Conversion (OTEC) uses the ocean thermal gradient between cooler deep and warmer shallow or surface seawaters to run a heat engine and produce useful work, usually in the form of electricity. OTEC can operate with a very high capacity factor and so can operate in base load mode.

Alkalinity is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.

In oceanic biogeochemistry, the solubility pump is a physico-chemical process that transports carbon as dissolved inorganic carbon (DIC) from the ocean's surface to its interior.
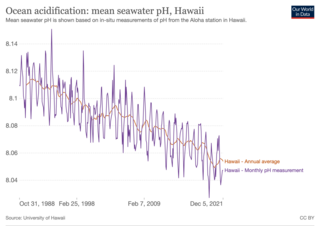
Ocean acidification is the reduction in the pH of the Earth’s ocean. This process takes place over periods lasting decades or more. Its main cause is the absorption of carbon dioxide (CO2) from the atmosphere. This, in turn, increases CO2 concentrations in the ocean. Between 23 and 30% of the CO2 that is in the atmosphere dissolves into oceans, rivers and lakes. Acidification is one of several effects of rising CO2 on the ocean. Other chemical changes to the ocean can also cause acidification. As the ocean absorbs CO2, seawater chemistry changes, which changes the living conditions of marine species. Many different species are affected, especially organisms that rely on calcium carbonate shells and skeletons, like mollusks, oysters and corals. Organisms like these struggle to build those parts of their anatomy when ocean waters have increased acidity.
Submarine groundwater discharge (SGD) is a hydrological process which commonly occurs in coastal areas. It is described as submarine inflow of fresh-, and brackish groundwater from land into the sea. Submarine Groundwater Discharge is controlled by several forcing mechanisms, which cause a hydraulic gradient between land and sea. Considering the different regional settings the discharge occurs either as (1) a focused flow along fractures in karst and rocky areas, (2) a dispersed flow in soft sediments, or (3) a recirculation of seawater within marine sediments. Submarine Groundwater Discharge plays an important role in coastal biogeochemical processes and hydrological cycles such as the formation of offshore plankton blooms, hydrological cycles, and the release of nutrients, trace elements and gases. It affects coastal ecosystems and has been used as a freshwater resource by some local communities for millennia.
The Global Ocean Data Analysis Project (GLODAP) is a synthesis project bringing together oceanographic data, featuring two major releases as of 2018. The central goal of GLODAP is to generate a global climatology of the World Ocean's carbon cycle for use in studies of both its natural and anthropogenically forced states. GLODAP is funded by the National Oceanic and Atmospheric Administration, the U.S. Department of Energy, and the National Science Foundation.
The Hawaii Ocean Time-series (HOT) program is a long-term oceanographic study based at the University of Hawaii at Manoa. In 2015, the American Society for Microbiology designated the HOT Program's field site Station ALOHA a "Milestone in Microbiology", for playing "a key role in defining the discipline of microbial oceanography and educating the public about the vital role of marine microbes in global ecosystems."
Marine chemistry, also known as ocean chemistry or chemical oceanography, is influenced by plate tectonics and seafloor spreading, turbidity currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology. The field of chemical oceanography studies the chemistry of marine environments including the influences of different variables. Marine life has adapted to the chemistries unique to earth's oceans, and marine ecosystems are sensitive to changes in ocean chemistry.

An algae scrubber is a water filtering device which uses light to grow algae; in this process, undesirable chemicals are removed from the water. Algae scrubbers allow saltwater, freshwater and pond hobbyists to operate their tanks using natural filtration in the form of primary production, much like oceans and lakes.
A mesocosm is any outdoor experimental system that examines the natural environment under controlled conditions. In this way mesocosm studies provide a link between field surveys and highly controlled laboratory experiments.

The oceanic carbon cycle is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic carbon and organic carbon. Part of the marine carbon cycle transforms carbon between non-living and living matter.
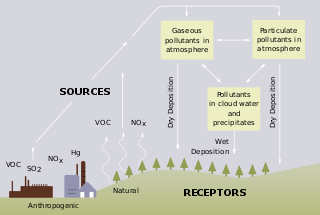
Freshwater acidification occurs when acidic inputs enter a body of fresh water through the weathering of rocks, invasion of acidifying gas, or by the reduction of acid anions, like sulfate and nitrate within the lake. Freshwater acidification is primarily caused by sulfur oxides (SOx) and nitrogen oxides (NOx) entering the water from atmospheric depositions and soil leaching. Carbonic acid and dissolved carbon dioxide can also enter freshwaters in a similar manner associated with runoff through carbon dioxide-rich soils. Runoff that contains these compounds may be accompanied by acidifying hydrogen ions and inorganic aluminum, which can be toxic to marine organisms. Acid rain is also a contributor to freshwater acidification. It is created when SOx and NOx react with water, oxygen, and other oxidants within the clouds.

The calcium cycle is a transfer of calcium between dissolved and solid phases. There is a continuous supply of calcium ions into waterways from rocks, organisms, and soils. Calcium ions are consumed and removed from aqueous environments as they react to form insoluble structures such as calcium carbonate and calcium silicate, which can deposit to form sediments or the exoskeletons of organisms. Calcium ions can also be utilized biologically, as calcium is essential to biological functions such as the production of bones and teeth or cellular function. The calcium cycle is a common thread between terrestrial, marine, geological, and biological processes. Calcium moves through these different media as it cycles throughout the Earth. The marine calcium cycle is affected by changing atmospheric carbon dioxide due to ocean acidification.

Ocean storage of carbon dioxide are technologies that aim to remove carbon dioxide from the atmosphere and safely store it in the oceans. Technology options include: dilute carbon dioxide injection and storage, release of solid carbon dioxide at depth, mineralization and deep sea sediments, carbon dioxide plumes, carbon dioxide lakes, changing mixing layers and several minimal impact methods.

The Arctic ocean covers an area of 14,056,000 squared kilometers, and supports a diverse and important socioeconomic food web of organisms, despite its average water temperature being 32 degrees Fahrenheit. Over the last three decades, the Arctic Ocean has experienced drastic changes due to climate change. One of the changes is in the acidity levels of the ocean, which have been consistently increasing at twice the rate of the Pacific and Atlantic oceans. Arctic Ocean acidification is a result of feedback from climate system mechanisms, and is having negative impacts on Arctic Ocean ecosystems and the organisms that live within them.

The European Project on Ocean Acidification (EPOCA) was Europe's first major research initiative and the first large-scale international research effort devoted to studying the impacts and consequences of ocean acidification. EPOCA was an EU FP7 Integrated Project active during four years, from 2008 to 2012.

Human activities affect marine life and marine habitats through overfishing, habitat loss, the introduction of invasive species, ocean pollution, ocean acidification and ocean warming. These impact marine ecosystems and food webs and may result in consequences as yet unrecognised for the biodiversity and continuation of marine life forms.

Jean-Pierre Gattuso is a French ocean scientist conducting research globally, from the pole to the tropics and from nearshore to the open ocean. His research addresses the biology of reef-building corals, the biogeochemistry of coastal ecosystems, and the response of marine plants, animals and ecosystems to global environmental change. He is also interested in transdisciplinary research, collaborating with social scientists to address ocean-based solutions to minimize climate change and its impacts. He is currently a CNRS Research Professor at Sorbonne University.

Particulate inorganic carbon (PIC) can be contrasted with dissolved inorganic carbon (DIC), the other form of inorganic carbon found in the ocean. These distinctions are important in chemical oceanography. Particulate inorganic carbon is sometimes called suspended inorganic carbon. In operational terms, it is defined as the inorganic carbon in particulate form that is too large to pass through the filter used to separate dissolved inorganic carbon.














