
A gas thermometer is a thermometer that measures temperature by the variation in volume or pressure of a gas. [1]

A gas thermometer is a thermometer that measures temperature by the variation in volume or pressure of a gas. [1]
This thermometer functions by Charles's Law. Charles's Law states that when the temperature of a gas increases, so does the volume. [2]
Using Charles's Law, the temperature can be measured by knowing the volume of gas at a certain temperature by using the formula, written below. Translating it to the correct levels of the device that is holding the gas. This works on the same principle as mercury thermometers.
or
is the volume,
is the thermodynamic temperature,
is the constant for the system.
is not a fixed constant across all systems and therefore needs to be found experimentally for a given system through testing with known temperature values.
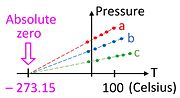
The constant volume gas thermometer plays a crucial role in understanding how absolute zero could be discovered long before the advent of cryogenics. Consider a graph of pressure versus temperature made not far from standard conditions (well above absolute zero) for three different samples of any ideal gas (a, b, c). To the extent that the gas is ideal, the pressure depends linearly on temperature, and the extrapolation to zero pressure occurs at absolute zero. [3] Note that data could have been collected with three different amounts of the same gas, which would have rendered this experiment easy to do in the eighteenth century.

Absolute zero is the lowest limit of the thermodynamic temperature scale, a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value, taken as zero kelvin. The fundamental particles of nature have minimum vibrational motion, retaining only quantum mechanical, zero-point energy-induced particle motion. The theoretical temperature is determined by extrapolating the ideal gas law; by international agreement, absolute zero is taken as −273.15 degrees on the Celsius scale, which equals −459.67 degrees on the Fahrenheit scale. The corresponding Kelvin and Rankine temperature scales set their zero points at absolute zero by definition.

In thermodynamics, an adiabatic process is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. Unlike an isothermal process, an adiabatic process transfers energy to the surroundings only as work. As a key concept in thermodynamics, the adiabatic process supports the theory that explains the first law of thermodynamics.

Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.

The thermodynamic free energy is a concept useful in the thermodynamics of chemical or thermal processes in engineering and science. The change in the free energy is the maximum amount of work that a thermodynamic system can perform in a process at constant temperature, and its sign indicates whether the process is thermodynamically favorable or forbidden. Since free energy usually contains potential energy, it is not absolute but depends on the choice of a zero point. Therefore, only relative free energy values, or changes in free energy, are physically meaningful.

Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics. A thermodynamic temperature reading of zero is of particular importance for the third law of thermodynamics. By convention, it is reported on the Kelvin scale of temperature in which the unit of measurement is the kelvin. For comparison, a temperature of 295 K is equal to 21.85 °C and 71.33 °F.

The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form:

An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics. The requirement of zero interaction can often be relaxed if, for example, the interaction is perfectly elastic or regarded as point-like collisions.

Charles's law is an experimental gas law that describes how gases tend to expand when heated. A modern statement of Charles's law is:
When the pressure on a sample of a dry gas is held constant, the Kelvin temperature and the volume will be in direct proportion.

The third law of thermodynamics states, regarding the properties of closed systems in thermodynamic equilibrium:
The entropy of a system approaches a constant value when its temperature approaches absolute zero.

The zeroth law of thermodynamics states that if two thermodynamic systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. Accordingly, thermal equilibrium between systems is a transitive relation.

The internal energy of a thermodynamic system is the energy contained within it. It is the energy necessary to create or prepare the system in its given internal state. It does not include the kinetic energy of motion of the system as a whole, nor the potential energy of the system as a whole due to external force fields, including the energy of displacement of the surroundings of the system. It keeps account of the gains and losses of energy of the system that are due to changes in its internal state. The internal energy cannot be measured directly. It is measured as a difference from a reference zero defined by a standard state. The difference is determined by thermodynamic processes that carry the system between the reference state and the given state of interest.
Gay-Lussac's law states that the pressure of a given mass of gas varies directly with the absolute temperature of the gas when the volume is kept constant. Mathematically, it can be written as: . It is a special case of the ideal gas law. Gay-Lussac is recognized for the Pressure Law which established that the pressure of an enclosed gas is directly proportional to its temperature and which he was the first to formulate. He is also sometimes credited with being the first to publish convincing evidence that shows the relationship between the pressure and temperature of a fixed mass of gas kept at a constant volume.

Thermodynamics is expressed by a mathematical framework of thermodynamic equations which relate various thermodynamic quantities and physical properties measured in a laboratory or production process. Thermodynamics is based on a fundamental set of postulates, that became the laws of thermodynamics.
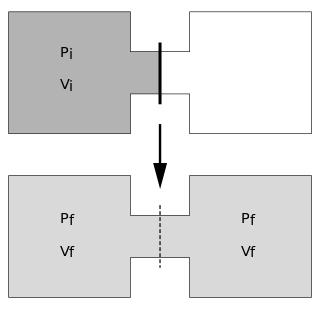
The Joule expansion is an irreversible process in thermodynamics in which a volume of gas is kept in one side of a thermally isolated container, with the other side of the container being evacuated. The partition between the two parts of the container is then opened, and the gas fills the whole container.

A thermodynamic instrument is any device which facilitates the quantitative measurement of thermodynamic systems. In order for a thermodynamic parameter to be truly defined, a technique for its measurement must be specified. For example, the ultimate definition of temperature is "what a thermometer reads". The question follows – what is a thermometer?
The concept entropy was first developed by German physicist Rudolf Clausius in the mid-nineteenth century as a thermodynamic property that predicts that certain spontaneous processes are irreversible or impossible. In statistical mechanics, entropy is formulated as a statistical property using probability theory. The statistical entropy perspective was introduced in 1870 by Austrian physicist Ludwig Boltzmann, who established a new field of physics that provided the descriptive linkage between the macroscopic observation of nature and the microscopic view based on the rigorous treatment of a large ensembles of microstates that constitute thermodynamic systems.

Gas is one of the four fundamental states of matter.

Temperature is a physical quantity that expresses how hot matter is, or as a measure of the average kinetic energy per atom or molecule in the system. It is the detectable portion of molar thermal energy which is present in all matter; a temperature diffence allows heat to occur, as energy flows from a hotter body to a colder body.

In thermodynamics, the volume of a system is an important extensive parameter for describing its thermodynamic state. The specific volume, an intensive property, is the system's volume per unit of mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure and temperature. For example, volume is related to the pressure and temperature of an ideal gas by the ideal gas law.
Scale of temperature is a methodology of calibrating the physical quantity temperature in metrology. Empirical scales measure temperature in relation to convenient and stable parameters, such as the freezing and boiling point of water. Absolute temperature is based on thermodynamic principles: using the lowest possible temperature as the zero point, and selecting a convenient incremental unit.