
Arabidopsis thaliana, the thale cress, mouse-ear cress or arabidopsis, is a small flowering plant native to Eurasia and Africa. A. thaliana is considered a weed; it is found along the shoulders of roads and in disturbed land.

In botany, a plant shoot consists of any plant stem together with its appendages, leaves and lateral buds, flowering stems, and flower buds. The new growth from seed germination that grows upward is a shoot where leaves will develop. In the spring, perennial plant shoots are the new growth that grows from the ground in herbaceous plants or the new stem or flower growth that grows on woody plants.

In vascular plants, the roots are the organs of a plant that are modified to provide anchorage for the plant and take in water and nutrients into the plant body, which allows plants to grow taller and faster. They are most often below the surface of the soil, but roots can also be aerial or aerating, that is, growing up above the ground or especially above water.

A vine is any plant with a growth habit of trailing or scandent stems, lianas or runners. The word vine can also refer to such stems or runners themselves, for instance, when used in wicker work.
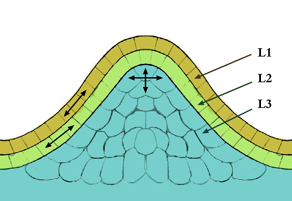
The meristem is a type of tissue found in plants. It consists of undifferentiated cells capable of cell division. Cells in the meristem can develop into all the other tissues and organs that occur in plants. These cells continue to divide until a time when they get differentiated and then lose the ability to divide.

Plant hormone are signal molecules, produced within plants, that occur in extremely low concentrations. Plant hormones control all aspects of plant growth and development, from embryogenesis, the regulation of organ size, pathogen defense, stress tolerance and through to reproductive development. Unlike in animals each plant cell is capable of producing hormones. Went and Thimann coined the term "phytohormone" and used it in the title of their 1937 book.

Vegetative reproduction is any form of asexual reproduction occurring in plants in which a new plant grows from a fragment or cutting of the parent plant or specialized reproductive structures, which are sometimes called vegetative propagules.
A maternal effect is a situation where the phenotype of an organism is determined not only by the environment it experiences and its genotype, but also by the environment and genotype of its mother. In genetics, maternal effects occur when an organism shows the phenotype expected from the genotype of the mother, irrespective of its own genotype, often due to the mother supplying messenger RNA or proteins to the egg. Maternal effects can also be caused by the maternal environment independent of genotype, sometimes controlling the size, sex, or behaviour of the offspring. These adaptive maternal effects lead to phenotypes of offspring that increase their fitness. Further, it introduces the concept of phenotypic plasticity, an important evolutionary concept. It has been proposed that maternal effects are important for the evolution of adaptive responses to environmental heterogeneity.

Trichomes ; from Ancient Greek τρίχωμα (tríkhōma) 'hair') are fine outgrowths or appendages on plants, algae, lichens, and certain protists. They are of diverse structure and function. Examples are hairs, glandular hairs, scales, and papillae. A covering of any kind of hair on a plant is an indumentum, and the surface bearing them is said to be pubescent.

Auxins are a class of plant hormones with some morphogen-like characteristics. Auxins play a cardinal role in coordination of many growth and behavioral processes in plant life cycles and are essential for plant body development. The Dutch biologist Frits Warmolt Went first described auxins and their role in plant growth in the 1920s. Kenneth V. Thimann became the first to isolate one of these phytohormones and to determine its chemical structure as indole-3-acetic acid (IAA). Went and Thimann co-authored a book on plant hormones, Phytohormones, in 1937.

Cytokinins (CK) are a class of plant hormones that promote cell division, or cytokinesis, in plant roots and shoots. They are involved primarily in cell growth and differentiation, but also affect apical dominance, axillary bud growth, and leaf senescence.

Plant callus is a growing mass of unorganized plant parenchyma cells. In living plants, callus cells are those cells that cover a plant wound. In biological research and biotechnology callus formation is induced from plant tissue samples (explants) after surface sterilization and plating onto tissue culture medium in vitro. The culture medium is supplemented with plant growth regulators, such as auxin, cytokinin, and gibberellin, to initiate callus formation or somatic embryogenesis. Callus initiation has been described for all major groups of land plants.

Phenotypic plasticity refers to some of the changes in an organism's behavior, morphology and physiology in response to a unique environment. Fundamental to the way in which organisms cope with environmental variation, phenotypic plasticity encompasses all types of environmentally induced changes that may or may not be permanent throughout an individual's lifespan.

A primordium in embryology, is an organ or tissue in its earliest recognizable stage of development. Cells of the primordium are called primordial cells. A primordium is the simplest set of cells capable of triggering growth of the would-be organ and the initial foundation from which an organ is able to grow. In flowering plants, a floral primordium gives rise to a flower.

Phytomorphology is the study of the physical form and external structure of plants. This is usually considered distinct from plant anatomy, which is the study of the internal structure of plants, especially at the microscopic level. Plant morphology is useful in the visual identification of plants. Recent studies in molecular biology started to investigate the molecular processes involved in determining the conservation and diversification of plant morphologies. In these studies transcriptome conservation patterns were found to mark crucial ontogenetic transitions during the plant life cycle which may result in evolutionary constraints limiting diversification.
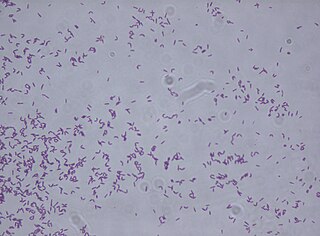
Rhodococcus fascians is a Gram positive bacterial phytopathogen that causes leafy gall disease. R. fascians is the only phytopathogenic member of the genus Rhodococcus; its host range includes both dicotyledonous and monocotyledonous hosts. Because it commonly afflicts tobacco (Nicotiana) plants, it is an agriculturally significant pathogen.

The evolution of plants has resulted in a wide range of complexity, from the earliest algal mats, through multicellular marine and freshwater green algae, terrestrial bryophytes, lycopods and ferns, to the complex gymnosperms and angiosperms of today. While many of the earliest groups continue to thrive, as exemplified by red and green algae in marine environments, more recently derived groups have displaced previously ecologically dominant ones; for example, the ascendance of flowering plants over gymnosperms in terrestrial environments.
Evolutionary developmental biology (evo-devo) is the study of developmental programs and patterns from an evolutionary perspective. It seeks to understand the various influences shaping the form and nature of life on the planet. Evo-devo arose as a separate branch of science rather recently. An early sign of this occurred in 1999.
Important structures in plant development are buds, shoots, roots, leaves, and flowers; plants produce these tissues and structures throughout their life from meristems located at the tips of organs, or between mature tissues. Thus, a living plant always has embryonic tissues. By contrast, an animal embryo will very early produce all of the body parts that it will ever have in its life. When the animal is born, it has all its body parts and from that point will only grow larger and more mature. However, both plants and animals pass through a phylotypic stage that evolved independently and that causes a developmental constraint limiting morphological diversification.
Cryptic mimicry is observed in animals as well as plants. In animals, this may involve nocturnality, camouflage, subterranean lifestyle, and mimicry. Generally, plant herbivores are visually oriented. So a mimicking plant should strongly resemble its host; this can be done through visual and/or textural change. Previous criteria for mimicry include similarity of leaf dimensions, leaf presentation, and intermodal distances between the host and mimicking plant.

















