
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vapourise, but when it does, a flame is a characteristic indicator of the reaction. While the activation energy must be overcome to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining. Combustion is often a complicated sequence of elementary radical reactions. Solid fuels, such as wood and coal, first undergo endothermic pyrolysis to produce gaseous fuels whose combustion then supplies the heat required to produce more of them. Combustion is often hot enough that incandescent light in the form of either glowing or a flame is produced. A simple example can be seen in the combustion of hydrogen and oxygen into water vapor, a reaction commonly used to fuel rocket engines. This reaction releases 242 kJ/mol of heat and reduces the enthalpy accordingly :

Fire is the rapid oxidation of a material in the exothermic chemical process of combustion, releasing heat, light, and various reaction products. Fire is hot because the conversion of the weak double bond in molecular oxygen, O2, to the stronger bonds in the combustion products carbon dioxide and water releases energy (418 kJ per 32 g of O2); the bond energies of the fuel play only a minor role here. At a certain point in the combustion reaction, called the ignition point, flames are produced. The flame is the visible portion of the fire. Flames consist primarily of carbon dioxide, water vapor, oxygen and nitrogen. If hot enough, the gases may become ionized to produce plasma. Depending on the substances alight, and any impurities outside, the color of the flame and the fire's intensity will be different.

Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species, either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system.
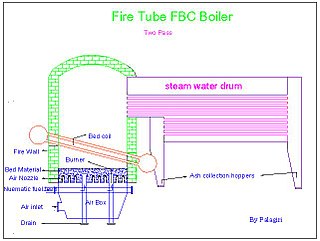
Fluidized bed combustion (FBC) is a combustion technology used to burn solid fuels.
A combustion chamber is part of an internal combustion engine in which the fuel/air mix is burned. For steam engines, the term has also been used for an extension of the firebox which is used to allow a more complete combustion process.

The fire triangle or combustion triangle is a simple model for understanding the necessary ingredients for most fires.
Thermofluids is a branch of science and engineering encompassing four intersecting fields:
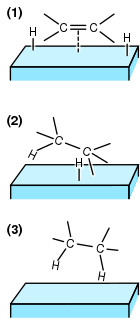
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present. Catalysts are useful because they increase the rate of a reaction without themselves being consumed and are therefore reusable.

A fluidized bed reactor (FBR) is a type of reactor device that can be used to carry out a variety of multiphase chemical reactions. In this type of reactor, a fluid is passed through a solid granular material at high enough speeds to suspend the solid and cause it to behave as though it were a fluid. This process, known as fluidization, imparts many important advantages to an FBR. As a result, FBRs are used for many industrial applications.

Chemical looping combustion (CLC) is a technological process typically employing a dual fluidized bed system. CLC operated with an interconnected moving bed with a fluidized bed system, has also been employed as a technology process. In CLC, a metal oxide is employed as a bed material providing the oxygen for combustion in the fuel reactor. The reduced metal is then transferred to the second bed and re-oxidized before being reintroduced back to the fuel reactor completing the loop. Fig 1 shows a simplified diagram of the CLC process. Fig 2 shows an example of a dual fluidized bed circulating reactor system and a moving bed-fluidized bed circulating reactor system.
The flame speed is the measured rate of expansion of the flame front in a combustion reaction. The flame is generally propagated spherically and the radial flame propagation velocity is defined as the flame speed. In other words, flame speed represents how rapidly the flame travels from an absolute reference point, while burning velocity presents the moving rate of chemical reactants into the reaction sheet from a local reference point located on the flame front.
Reactive flash volatilization (RFV) is a chemical process that rapidly converts nonvolatile solids and liquids to volatile compounds by thermal decomposition for integration with catalytic chemistries.
Self-propagating high-temperature synthesis (SHS) is a method for producing both inorganic and organic compounds by exothermic combustion reactions in solids of different nature. Reactions can occur between a solid reactant coupled with either a gas, liquid, or other solid. If the reactants, intermediates, and products are all solids, it is known as a solid flame. If the reaction occurs between a solid reactant and a gas phase reactant, it is called infiltration combustion. Since the process occurs at high temperatures, the method is ideally suited for the production of refractory materials including powders, metallic alloys, or ceramics.
Micro-combustion is the sequence of exothermic chemical reaction between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species at micro level. The release of heat can result in the production of light in the form of either glowing or a flame. Fuels of interest often include organic compounds in the gas, liquid or solid phase. The major problem of micro-combustion is the high surface to volume ratio. As the surface to volume ratio increases heat loss to walls of combustor increases which leads to flame quenching.
Laminar flame speed is an intrinsic characteristic of premixed combustible mixtures that plays a key role in understanding a mixture’s reactivity, diffusivity, and exothermicity. It is the speed at which an un-stretched laminar flame will propagate through a quiescent mixture of unburned reactants. Laminar flame speed is defined as the normal component of velocity of flame relative to unburned gas. Laminar flame speed is given the symbol sL. According to the thermal flame theory of Mallard and Le Chatelier, the un-stretched laminar flame speed is dependent on only three properties of a chemical mixture: the thermal diffusivity of the mixture, the reaction rate of the mixture and the temperature through the flame zone:

Piobert's law applies to the reaction of solid propellant grains to generate hot gas. It is stated: "Burning takes place by parallel layers where the surface of the grain regresses, layer by layer, normal to the surface at every point."

Aerogel is a synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas without significant collapse of the gel structure. The result is a solid with extremely low density and extremely low thermal conductivity. Nicknames include frozen smoke, solid smoke, solid air, solid cloud, and blue smoke, owing to its translucent nature and the way light scatters in the material. Silica aerogels feel like fragile expanded polystyrene to the touch, while some polymer-based aerogels feel like rigid foams. Aerogels can be made from a variety of chemical compounds.
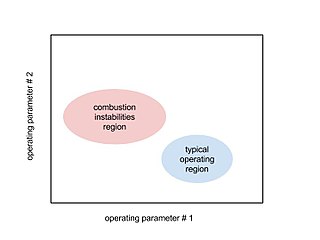
Combustion instabilities are physical phenomena occurring in a reacting flow in which some perturbations, even very small ones, grow and then become large enough to alter the features of the flow in some particular way.

Ashwani K. Gupta is a British-American engineer and educator with research focus on combustion, fuels, fuel reforming, advanced diagnostics, High Temperature Air Combustion, and high-intensity distributed combustion, green combustion turbine, micro-combustion, and air pollution. He is an Distinguished University Professor at the University of Maryland. Gupta is also Professor of Mechanical Engineering at the University of Maryland and Director of Combustion Laboratory. He is also an Affiliate Professor at Institute of Physical Science and Technology, University of Maryland which is part of the University of Maryland College of Computer, Mathematical and Natural Sciences.
Chemical looping reforming (CLR) and gasification (CLG) are the operations that involve the use of gaseous carbonaceous feedstock and solid carbonaceous feedstock, respectively, in their conversion to syngas in the chemical looping scheme. The typical gaseous carbonaceous feedstocks used are natural gas and reducing tail gas, while the typical solid carbonaceous feedstocks used are coal and biomass. The feedstocks are partially oxidized to generate syngas using metal oxide oxygen carriers as the oxidant. The reduced metal oxide is then oxidized in the regeneration step using air. The syngas is an important intermediate for generation of such diverse products as electricity, chemicals, hydrogen, and liquid fuels.













