
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.

In physiology, an action potential (AP) occurs when the membrane potential of a specific cell location rapidly rises and falls: this depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, endocrine cells, glomus cells, and in some plant cells.

The contraction of cardiac muscle in all animals is initiated by electrical impulses known as action potentials. The rate at which these impulses fire, controls the rate of cardiac contraction, that is, the heart rate. The cells that create these rhythmic impulses, setting the pace for blood pumping, are called pacemaker cells, and they directly control the heart rate. They make up the cardiac pacemaker, that is, the natural pacemaker of the heart. In most humans, the concentration of pacemaker cells in the sinoatrial (SA) node is the natural pacemaker, and the resultant rhythm is a sinus rhythm.
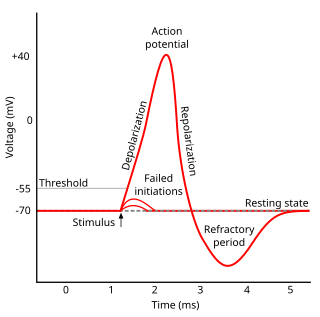
Refractoriness is the fundamental property of any object of autowave nature not to respond on stimuli, if the object stays in the specific refractory state. In common sense, refractory period is the characteristic recovery time, a period that is associated with the motion of the image point on the left branch of the isocline .
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. IPSP were first investigated in motorneurons by David P. C. Lloyd, John Eccles and Rodolfo Llinás in the 1950s and 1960s. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell to cell signalling. Inhibitory presynaptic neurons release neurotransmitters that then bind to the postsynaptic receptors; this induces a change in the permeability of the postsynaptic neuronal membrane to particular ions. An electric current that changes the postsynaptic membrane potential to create a more negative postsynaptic potential is generated, i.e. the postsynaptic membrane potential becomes more negative than the resting membrane potential, and this is called hyperpolarisation. To generate an action potential, the postsynaptic membrane must depolarize—the membrane potential must reach a voltage threshold more positive than the resting membrane potential. Therefore, hyperpolarisation of the postsynaptic membrane makes it less likely for depolarisation to sufficiently occur to generate an action potential in the postsynaptic neurone.

In neuroscience, an excitatory postsynaptic potential (EPSP) is a postsynaptic potential that makes the postsynaptic neuron more likely to fire an action potential. This temporary depolarization of postsynaptic membrane potential, caused by the flow of positively charged ions into the postsynaptic cell, is a result of opening ligand-gated ion channels. These are the opposite of inhibitory postsynaptic potentials (IPSPs), which usually result from the flow of negative ions into the cell or positive ions out of the cell. EPSPs can also result from a decrease in outgoing positive charges, while IPSPs are sometimes caused by an increase in positive charge outflow. The flow of ions that causes an EPSP is an excitatory postsynaptic current (EPSC).
Hyperpolarization is a change in a cell's membrane potential that makes it more negative. It is the opposite of a depolarization. It inhibits action potentials by increasing the stimulus required to move the membrane potential to the action potential threshold.
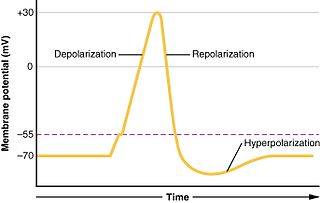
In biology, depolarization is a change within a cell, during which the cell undergoes a shift in electric charge distribution, resulting in less negative charge inside the cell. Depolarization is essential to the function of many cells, communication between cells, and the overall physiology of an organism.
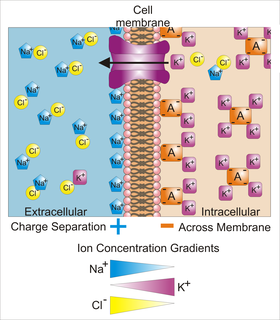
Membrane potential is the difference in electric potential between the interior and the exterior of a biological cell. For the exterior of the cell, typical values of membrane potential, normally given in units of millivolts and denoted as mV, range from –40 mV to –80 mV.

In electrophysiology, the threshold potential is the critical level to which a membrane potential must be depolarized to initiate an action potential. In neuroscience, threshold potentials are necessary to regulate and propagate signaling in both the central nervous system (CNS) and the peripheral nervous system (PNS).

The axon hillock is a specialized part of the cell body of a neuron that connects to the axon. It can be identified using light microscopy from its appearance and location in a neuron and from its sparse distribution of Nissl substance.
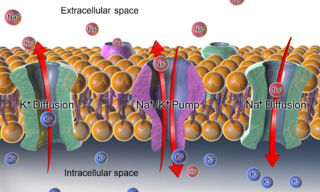
The relatively static membrane potential of quiescent cells is called the resting membrane potential, as opposed to the specific dynamic electrochemical phenomena called action potential and graded membrane potential.
The cardiac action potential is a brief change in voltage across the cell membrane of heart cells. This is caused by the movement of charged atoms between the inside and outside of the cell, through proteins called ion channels. The cardiac action potential differs from action potentials found in other types of electrically excitable cells, such as nerves. Action potentials also vary within the heart; this is due to the presence of different ion channels in different cells.
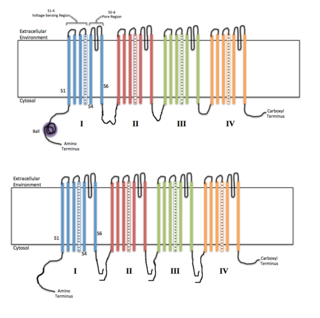
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions have been identified. The opening and closing of the channels are triggered by changing ion concentration, and hence charge gradient, between the sides of the cell membrane.
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's plasma membrane. They belong to the superfamily of cation channels and can be classified according to the trigger that opens the channel for such ions, i.e. either a voltage-change ("voltage-gated", "voltage-sensitive", or "voltage-dependent" sodium channel; also called "VGSCs" or "Nav channel") or a binding of a substance (a ligand) to the channel (ligand-gated sodium channels).
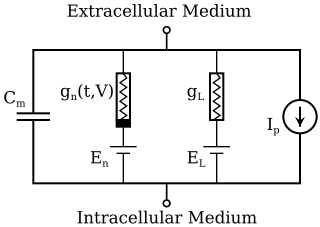
The Hodgkin–Huxley model, or conductance-based model, is a mathematical model that describes how action potentials in neurons are initiated and propagated. It is a set of nonlinear differential equations that approximates the electrical characteristics of excitable cells such as neurons and cardiac myocytes. It is a continuous-time dynamical system.
Bathmotropic often refers to modifying the degree of excitability specifically of the heart; in general, it refers to modification of the degree of excitability of musculature in general, including the heart. It especially is used to describe the effects of the cardiac nerves on cardiac excitability. Positive bathmotropic effects increase the response of muscle to stimulation, whereas negative bathmotropic effects decrease the response of muscle to stimulation. In a whole, it is the heart's reaction to catecholamines. Conditions that decrease bathmotropy cause the heart to be less responsive to catecholaminergic drugs. A substance that has a bathmotropic effect is known as a bathmotrope.
Cellular neuroscience is a branch of neuroscience concerned with the study of neurons at a cellular level. This includes morphology and physiological properties of single neurons. Several techniques such as intracellular recording, patch-clamp, and voltage-clamp technique, pharmacology, confocal imaging, molecular biology, two photon laser scanning microscopy and Ca2+ imaging have been used to study activity at the cellular level. Cellular neuroscience examines the various types of neurons, the functions of different neurons, the influence of neurons upon each other, and how neurons work together.
A depolarizing prepulse (DPP) is an electrical stimulus that causes the potential difference measured across a neuronal membrane to become more positive or less negative, and precedes another electrical stimulus. DPPs may be of either the voltage or current stimulus variety and have been used to inhibit neural activity, selectively excite neurons, and increase the pain threshold associated with electrocutaneous stimulation.
A paroxysmal depolarizing shift (PDS) or depolarizing shift is a hallmark of cellular manifestation of epilepsy. Little is known about the initiation, propagation and termination of PDS. Previously, electrophysiological studies have provided the evidence that there is a Ca2+ mediated depolarization, which causes voltage gated Na+ channels to open, resulting in action potentials. This depolarization is followed by a period of hyperpolarization mediated by Ca2+-dependent K+ channels or GABA-activated Cl− influx.. In general, synaptic PDS could be initiated by EPSPs, and the plateau potential of the PDS is maintained by a combination of synaptic potentials (EPSPs, IPSPs) and ionic conductances (persistent sodium current and high-threshold calcium current) and the post-PDS hyperpolarization is governed by multiple potassium currents, activated by calcium or sodium entry, as well as by leak current. The next cycle of depolarization is initiated by both synaptic drive and the hyperpolarization-activated IH current.












