
Molecular engineering is an emerging field of study concerned with the design and testing of molecular properties, behavior and interactions in order to assemble better materials, systems, and processes for specific functions. This approach, in which observable properties of a macroscopic system are influenced by direct alteration of a molecular structure, falls into the broader category of “bottom-up” design.

Gas mixtures can be effectively separated by synthetic membranes made from polymers such as polyamide or cellulose acetate, or from ceramic materials.

Metal–organic frameworks (MOFs) are a class of porous polymers consisting of metal clusters coordinated to organic ligands to form one-, two-, or three-dimensional structures. The organic ligands included are sometimes referred to as "struts" or "linkers", one example being 1,4-benzenedicarboxylic acid (BDC).

Zeolitic imidazolate frameworks (ZIFs) are a class of metal-organic frameworks (MOFs) that are topologically isomorphic with zeolites. ZIF glasses can be synthesized by the melt-quench method, and the first melt-quenched ZIF glass was firstly made and reported by Bennett et al. back in 2015. ZIFs are composed of tetrahedrally-coordinated transition metal ions connected by imidazolate linkers. Since the metal-imidazole-metal angle is similar to the 145° Si-O-Si angle in zeolites, ZIFs have zeolite-like topologies. As of 2010, 105 ZIF topologies have been reported in the literature. Due to their robust porosity, resistance to thermal changes, and chemical stability, ZIFs are being investigated for applications such as carbon dioxide capture.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.
Covalent organic frameworks (COFs) are a class of porous polymers that form two- or three-dimensional structures through reactions between organic precursors resulting in strong, covalent bonds to afford porous, stable, and crystalline materials. COFs emerged as a field from the overarching domain of organic materials as researchers optimized both synthetic control and precursor selection. These improvements to coordination chemistry enabled non-porous and amorphous organic materials such as organic polymers to advance into the construction of porous, crystalline materials with rigid structures that granted exceptional material stability in a wide range of solvents and conditions. Through the development of reticular chemistry, precise synthetic control was achieved and resulted in ordered, nano-porous structures with highly preferential structural orientation and properties which could be synergistically enhanced and amplified. With judicious selection of COF secondary building units (SBUs), or precursors, the final structure could be predetermined, and modified with exceptional control enabling fine-tuning of emergent properties. This level of control facilitates the COF material to be designed, synthesized, and utilized in various applications, many times with metrics on scale or surpassing that of the current state-of-the-art approaches.
The electrochemical reduction of carbon dioxide, also known as CO2RR, is the conversion of carbon dioxide to more reduced chemical species using electrical energy. It represents one potential step in the broad scheme of carbon capture and utilization.
In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent.

Hong-Cai (Joe) Zhou is a Chinese–American chemist and academic. He is the Davidson Professor of Science and Robert A. Welch Chair in Chemistry at Texas A&M University. He is the associate editor of the journal Inorganic Chemistry.
Niveen M. Khashab is a Lebanese chemist and an associate Professor of chemical Sciences and engineering at King Abdullah University of Science and Technology in Saudi Arabia since 2009. She is a laureate of the 2017 L'Oréal-UNESCO Awards for Women in Science "for her contributions to innovative smart hybrid materials aimed at drug delivery and for developing new techniques to monitor intracellular antioxidant activity." She is also a fellow of the Royal Chemical Society, and a member of the American Chemical Society.
Laura Maria Herz is a professor of physics at the University of Oxford. She works on femtosecond spectroscopy for the analysis of semiconductor materials.
Rahul Banerjee is a Bengali Indian organic chemist and a professor at the department of chemical sciences of the Indian Institute of Science Education and Research, Kolkata. Banerjee, a fellow of the Royal Society of Chemistry, is known for his studies in the field of Metal–organic framework designing. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, for his contributions to chemical sciences in 2018. Currently he is one of the associate editor of international peer-review journal Journal of the American Chemical Society.
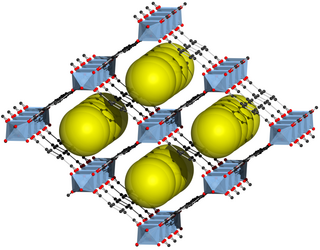
MIL-53 belongs to the class of metal-organic framework (MOF) materials. The first synthesis and the name was established by the group of Gérard Férey in 2002. The MIL-53 structure consists of inorganic [M-OH] chains, which are connected to four neighboring inorganic chains by therephthalate-based linker molecules. Each metal center is octahedrally coordinated by six oxygen atoms. Four of these oxygen atoms originate from four different carboxylate groups and the remaining two oxygen atoms belong to two different μ-OH moieties, which bridge neighboring metal centers. The resulting framework structure contains one-dimensional diamond-shaped pores. Many research group have investigated the flexibility of the MIL-53 structure. This flexible behavior, during which the pore cross-section changes reversibly, was termed 'breathing.effect' and describes the ability of the MIL-53 framework to respond to external stimuli.
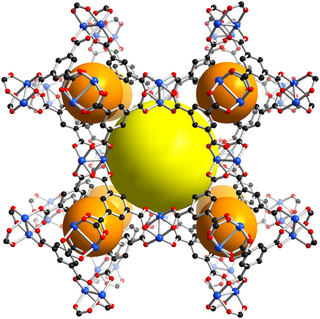
HKUST-1, which is also called MOF-199, is a material in the class of metal-organic frameworks (MOFs). Metal-organic frameworks are crystalline materials, in which metals are linked by ligands to form repeating coordination motives extending in three dimensions. The HKUST-1 framework is built up of dimeric metal units, which are connected by benzene-1,3,5-tricarboxylate linker molecules. The paddlewheel unit is the commonly used structural motif to describe the coordination environment of the metal centers and also called secondary building unit (SBU) of the HKUST-1 structure. The paddlewheel is built up of four benzene-1,3,5-tricarboxylate linkers molecules, which bridge two metal centers. One water molecules is coordinated to each of the two metal centers at the axial position of the paddlewheel unit in the hydrated state, which is usually found if the material is handled in air. After an activation process, these water molecules can be removed and the coordination site at the metal atoms is left unoccupied. This unoccupied coordination site is called coordinatively unsaturated site (CUS) and can be accessed by other molecules.

Conductive metal−organic frameworks are a class of metal–organic frameworks with intrinsic ability of electronic conduction. Metal ions and organic linker self-assemble to form a framework which can be 1D/2D/3D in connectivity. The first conductive MOF, Cu[Cu(2,3-pyrazinedithiol)2] was described in 2009 and exhibited electrical conductivity of 6 × 10−4 S cm−1 at 300 K.

Kenneth J. Balkus Jr. is an American chemist and materials scientist. He is professor of chemistry and former department chair at The University of Texas at Dallas. He is a Fellow of the American Chemical Society and a recipient of the ACS Doherty Award. His well known work is synthesis of zeolite UTD-1, the first high-silica zeolite to contain a one-dimensional, extra-large 14-ring pore system. Other notable work include rare-earth metal organic frameworks. He is editor to Journal of Porous Materials, Springer. He is also co-founder of DB Therapeutics, a company developing cancer therapies.
Connie C. Lu is a Taiwanese-American inorganic chemist and a professor of chemistry at the University of Bonn. She was previously a professor of chemistry at the University of Minnesota, Twin Cities. Lu's research focuses on the synthesis of novel bimetallic coordination complexes, as well as metal-organic frameworks. These molecules and materials are investigated for the catalytic conversion of small molecules like as N2 and CO2 into value-added chemicals like ammonia and methanol. Lu is the recipient of multiple awards for her research, including the National Science Foundation CAREER Award and the Sloan Research Fellowship in 2013, and an Early Career Award from the University of Minnesota's Initiative for Renewable Energy and the Environment in 2010.
Carboxylate–based metal–organic frameworks are metal–organic frameworks that are based on organic molecules comprising carboxylate functional groups.
Amanda Morris is an American chemist who is the Patricia Caldwell Faculty Fellow and professor of inorganic and energy chemistry at Virginia Tech. Her research considers next-generation materials for catalysis and light-harvesting. She was elected chair of the American Chemical Society Gay and Transgender Chemists and Allies committee in 2021.
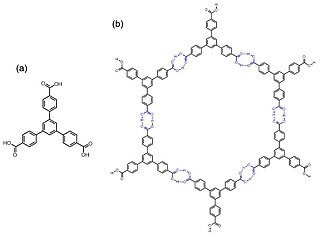
Hydrogen-bonded organic frameworks (HOFs) are a class of porous polymers formed by hydrogen bonds among molecular monomer units to afford porosity and structural flexibility. There are diverse hydrogen bonding pair choices that could be used in HOFs construction, including identical or nonidentical hydrogen bonding donors and acceptors. For organic groups acting as hydrogen bonding units, species like carboxylic acid, amide, 2,4-diaminotriazine, and imidazole, etc., are commonly used for the formation of hydrogen bonding interaction. Compared with other organic frameworks, like COF and MOF, the binding force of HOFs is relatively weaker, and the activation of HOFs is more difficult than other frameworks, while the reversibility of hydrogen bonds guarantees a high crystallinity of the materials. Though the stability and pore size expansion of HOFs has potential problems, HOFs still show strong potential for applications in different areas.












