Related Research Articles

Monoclonal antibodies are antibodies that are made by identical immune cells that are all clones of a unique parent cell. Monoclonal antibodies can have monovalent affinity, in that they bind to the same epitope. In contrast, polyclonal antibodies bind to multiple epitopes and are usually made by several different plasma cell lineages. Bispecific monoclonal antibodies can also be engineered, by increasing the therapeutic targets of one single monoclonal antibody to two epitopes.

The dermis or corium is a layer of skin between the epidermis and subcutaneous tissues, that primarily consists of dense irregular connective tissue and cushions the body from stress and strain. It is divided into two layers, the superficial area adjacent to the epidermis called the papillary region and a deep thicker area known as the reticular dermis. The dermis is tightly connected to the epidermis through a basement membrane. Structural components of the dermis are collagen, elastic fibers, and extrafibrillar matrix. It also contains mechanoreceptors that provide the sense of touch and thermoreceptors that provide the sense of heat. In addition, hair follicles, sweat glands, sebaceous glands, apocrine glands, lymphatic vessels, nerves and blood vessels are present in the dermis. Those blood vessels provide nourishment and waste removal for both dermal and epidermal cells.

A bioreactor refers to any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from litres to cubic metres, and are often made of stainless steel.
Cell culture is the process by which cells are grown under controlled conditions, generally outside their natural environment. After the cells of interest have been isolated from living tissue, they can subsequently be maintained under carefully controlled conditions. These conditions vary for each cell type, but generally consist of a suitable vessel with a substrate or medium that supplies the essential nutrients (amino acids, carbohydrates, vitamins, minerals), growth factors, hormones, and gases (CO2, O2), and regulates the physio-chemical environment (pH buffer, osmotic pressure, temperature). Most cells require a surface or an artificial substrate (adherent or monolayer culture) whereas others can be grown free floating in culture medium (suspension culture). The lifespan of most cells is genetically determined, but some cell culturing cells have been “transformed” into immortal cells which will reproduce indefinitely if the optimal conditions are provided.

Hybridoma technology is a method for producing large numbers of identical antibodies. This process starts by injecting a mouse with an antigen that provokes an immune response. A type of white blood cell, the B cell, produces antibodies that bind to the injected antigen. These antibody producing B-cells are then harvested from the mouse and, in turn, fused with immortal B cell cancer cells, a myeloma, to produce a hybrid cell line called a hybridoma, which has both the antibody-producing ability of the B-cell and the longevity and reproductivity of the myeloma. The hybridomas can be grown in culture, each culture starting with one viable hybridoma cell, producing cultures each of which consists of genetically identical hybridomas which produce one antibody per culture (monoclonal) rather than mixtures of different antibodies (polyclonal). The myeloma cell line that is used in this process is selected for its ability to grow in tissue culture and for an absence of antibody synthesis. In contrast to polyclonal antibodies, which are mixtures of many different antibody molecules, the monoclonal antibodies produced by each hybridoma line are all chemically identical.
A biopharmaceutical, also known as a biologic(al) medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living cells used in cell therapy. Biologics can be composed of sugars, proteins, or nucleic acids or complex combinations of these substances, or may be living cells or tissues. They are isolated from living sources—human, animal, plant, fungal, or microbial.
Industrial fermentation is the intentional use of fermentation by microorganisms such as bacteria and fungi as well as eukaryotic cells like CHO cells and insect cells, to make products useful to humans. Fermented products have applications as food as well as in general industry. Some commodity chemicals, such as acetic acid, citric acid, and ethanol are made by fermentation. The rate of fermentation depends on the concentration of microorganisms, cells, cellular components, and enzymes as well as temperature, pH and for aerobic fermentation oxygen. Product recovery frequently involves the concentration of the dilute solution. Nearly all commercially produced enzymes, such as lipase, invertase and rennet, are made by fermentation with genetically modified microbes. In some cases, production of biomass itself is the objective, as in the case of baker's yeast and lactic acid bacteria starter cultures for cheesemaking. In general, fermentations can be divided into four types:

Influenza C virus is the species in the genus Influenzavirus C, in the virus family Orthomyxoviridae, which like other influenza viruses, causes influenza.

Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system actively lyses a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.
A bioartificial liver device (BAL) is an artificial extracorporeal supportive device for an individual who is suffering from acute liver failure.
Fed-batch culture is, in the broadest sense, defined as an operational technique in biotechnological processes where one or more nutrients (substrates) are fed (supplied) to the bioreactor during cultivation and in which the product(s) remain in the bioreactor until the end of the run. An alternative description of the method is that of a culture in which "a base medium supports initial cell culture and a feed medium is added to prevent nutrient depletion". It is also a type of semi-batch culture. In some cases, all the nutrients are fed into the bioreactor. The advantage of the fed-batch culture is that one can control concentration of fed-substrate in the culture liquid at arbitrarily desired levels.

Interferon-induced transmembrane protein 1 is a protein that in humans is encoded by the IFITM1 gene. IFITM1 has also recently been designated CD225. This protein has several additional names: fragilis, IFI17 [interferon-induced protein 17], 9-27 [Interferon-inducible protein 9-27] and Leu13.
Membrane bioreactor (MBR) is the combination of a membrane process like microfiltration or ultrafiltration with a biological wastewater treatment process, the activated sludge process. It is now widely used for municipal and industrial wastewater treatment.
CR6261 is a monoclonal antibody that binds to a broad range of the influenza virus including the 1918 "Spanish flu" (SC1918/H1) and to a virus of the H5N1 class of avian influenza that jumped from chickens to a human in Vietnam in 2004 (Viet04/H5). In contrast to most antibodies generated by exposure to influenza, which can only neutralize a few strains from within a single virus subtype, CR6261 neutralizes numerous strains from multiple subtypes. CR6261 recognizes a highly conserved helical region in the membrane-proximal stem of hemagglutinin, the predominant protein on the surface of the influenza virus. Based upon the conservation of the amino acid sequence on this part of hemagglutinin, CR6261 is predicted to neutralize roughly 50% of all flu viruses. It was found by The Scripps Research Institute and the Dutch biopharmaceutical company, Crucell.
Virus quantification involves counting the number of viruses in a specific volume to determine the virus concentration. It is utilized in both research and development (R&D) in commercial and academic laboratories as well as production situations where the quantity of virus at various steps is an important variable. For example, the production of viral vaccines, recombinant proteins using viral vectors and viral antigens all require virus quantification to continually adapt and monitor the process in order to optimize production yields and respond to ever changing demands and applications. Examples of specific instances where known viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization and adaptation of methods to cell culture. This page discusses various techniques currently used to quantify viruses in liquid samples. These methods are separated into two categories, traditional vs. modern methods. Traditional methods are industry-standard methods that have been used for decades but are generally slow and labor-intensive. Modern methods are relatively new commercially available products and kits that greatly reduce quantification time. This is not meant to be an exhaustive review of all potential methods, but rather a representative cross-section of traditional methods and new, commercially available methods. While other published methods may exist for virus quantification, non-commercial methods are not discussed here.

Dundee Cell Products (DCP) is a biotechnology company headquartered in Dundee, Scotland, United Kingdom. The company is a bioreagents and life sciences services company which commercialises research tools for biochemistry, molecular biology and cell biology research, and provides services in these areas to the life sciences community. The company’s key business activities include research and development of new innovative products and services, commercialization of products in-licensed from academic institutions, distribution of life sciences research products from commercial partners and supply of contract research services to both academic and pharmaceutical/biotechnology companies customers.
A rabbit hybridoma is a hybrid cell line formed by the fusion of an antibody producing rabbit B cell with a cancerous B-cell (myeloma).
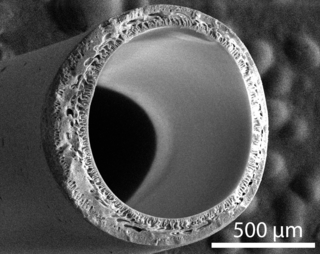
Hollow fiber membranes (HFMs) are a class of artificial membranes containing a semi-permeable barrier in the form of a hollow fiber. Originally developed in the 1960s for reverse osmosis applications, hollow fiber membranes have since become prevalent in water treatment, desalination, cell culture, medicine, and tissue engineering. Most commercial hollow fiber membranes are packed into cartridges which can be used for a variety of liquid and gaseous separations.
Recombinant antibodies are antibody fragments produced by using recombinant antibody coding genes. They mostly consist of a heavy and light chain of the variable region of immunoglobulin. Recombinant antibodies have many advantages in both medical and research applications, which make them a popular subject of exploration and new production against specific targets. The most commonly used form is the single chain variable fragment (scFv), which has shown the most promising traits exploitable in human medicine and research. In contrast to monoclonal antibodies produced by hybridoma technology, which may lose the capacity to produce the desired antibody over time or the antibody may undergo unwanted changes, which affect its functionality, recombinant antibodies produced in phage display maintain high standard of specificity and low immunogenicity.
mAb114 is a monoclonal antibody that is being evaluated as a treatment for Ebola virus disease. Its discovery was led by the laboratory of Nancy Sullivan at the United States National Institute of Health Vaccine Research Center and J. J. Muyembe-Tamfum from the Institut National pour la Recherche Biomedicale (INRB) in the Democratic Republic of Congo, working in collaboration with the Institute of Biomedical Research and the Unites States Army Medical Research Institute of Infectious Diseases. mAb114 was isolated from the blood of a survivor of the 1995 outbreak of Ebola virus disease in Kikwit, Democratic Republic of Congo roughly ten years later.
References
- ↑ Hirschel M, Gangemi JD, McSharry J., Myers C. Novel Uses for Hollow Fiber Bioreactors Genetic Engineering News Jun 15, 2011 (Vol. 31, No. 12).
- ↑ Knazek RA, Gullino PM, Kohler PO, Dedrick RL. Cell culture on artificial capillaries: an approach to tissue growth in vitro. Science. 1972 Oct 6;178(4056):65-6.
- ↑ Cell culture on semi-permeable tubular membranes U.S. Patent US 3821087 A
- ↑ Ahern, H. Hollow Fiber Bioreactor Systems Increase Cell Culture Yield The Scientist Magazine (1990)
- ↑ Extra-capillary fluid cycling system and method for a cell culture. U.S. Patent US 20130058907 A1
- ↑ Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495.
- ↑ Gramer, MJ. Britton TL. Selection and Isolation of Cells for Optimal Growth in Hollow Fiber Bioreactors Hybridoma 2000. 19(5):407-412.
- ↑ De Napoli, Ilaria E.; Zanetti, Elisabetta M.; Fragomeni, Gionata; Giuzio, Ermenegildo; Audenino, Alberto L.; Catapano, Gerardo (2014). "Transport modeling of convection-enhanced hollow fiber membrane bioreactors for therapeutic applications". Journal of Membrane Science. 471: 347–361. doi:10.1016/j.memsci.2014.08.026.
- ↑ Tapia, F. et al. Production of high-titer human influenza A virus with adherent and suspension MDCK cells cultured in a single-use hollow fiber bioreactor Vaccine 32 (2014): 1003-1011.