The term human equivalent is used in a number of different contexts. This term can refer to human equivalents of various comparisons of animate and inanimate things.
Animal models are used to learn more about a disease, its diagnosis and its treatment, with animal models predicting human toxicity in up to 71% of cases. [1] The human equivalent dose (HED) or human equivalent concentration (HEC) is the quantity of a chemical that, when administered to humans, produces an effect equal to that produced in test animals by a smaller dose. [2] Calculating the HED is a step in carrying out a clinical trial of a pharmaceutical drug. [3]
The concept of human-equivalent energy (H-e) assists in understanding of energy flows in physical and biological systems by expressing energy units in human terms: it provides a “feel” for the use of a given amount of energy by expressing it in terms of the relative quantity of energy needed for human metabolism, [4] assuming an average human energy expenditure of 12,500 kJ per day and a basal metabolic rate of 80 watts. [5] A light bulb running at 100 watts is running at 1.25 human equivalents (100/80), i.e. 1.25 H-e. On the other hand, a human may generate as much as 1,000 watts for a task lasting a few minutes, or even more for a task of a few seconds' duration, while climbing a flight of stairs may represent work at a rate of about 200 watts. [6]
The ages of domestic cats and dogs are often referred to in terms of "cat years" or "dog years", representing a conversion to human-equivalent years. One formula for cat years is based on a cat reaching maturity in approximately 1 year, which could be seen as 16 in human terms, then adding about 4 years for every year the cat ages. A 5-year-old cat would then be (5 − 1) × 4 + 16 = 32 "cat years" (i.e. human-equivalent years), and a 10-year-old cat (10 − 1) × 4 + 16 = 52 in human terms. [7]
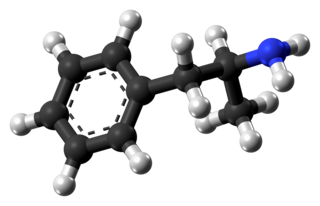
Amphetamine is a strong central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. Amphetamine was discovered in 1887 and exists as two enantiomers: levoamphetamine and dextroamphetamine. Amphetamine properly refers to a specific chemical, the racemic free base, which is equal parts of the two enantiomers in their pure amine forms. The term is frequently used informally to refer to any combination of the enantiomers, or to either of them alone. Historically, it has been used to treat nasal congestion and depression. Amphetamine is also used as an athletic performance enhancer and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. It is a prescription drug in many countries, and unauthorized possession and distribution of amphetamine are often tightly controlled due to the significant health risks associated with recreational use.

Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous molecule which exerts a biochemical or physiological effect on the cell, tissue, organ, or organism. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.

The sievert is a unit in the International System of Units (SI) intended to represent the stochastic health risk of ionizing radiation, which is defined as the probability of causing radiation-induced cancer and genetic damage. The sievert is important in dosimetry and radiation protection. It is named after Rolf Maximilian Sievert, a Swedish medical physicist renowned for work on radiation dose measurement and research into the biological effects of radiation.
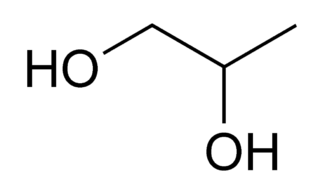
Propylene glycol (IUPAC name: propane-1,2-diol) is a viscous, colorless liquid, which is nearly odorless but possesses a faintly sweet taste. Its chemical formula is CH3CH(OH)CH2OH. Containing two alcohol groups, it is classed as a diol. It is miscible with a broad range of solvents, including water, acetone, and chloroform. In general, glycols are non-irritating and have very low volatility.
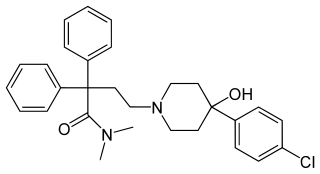
Loperamide, sold under the brand name Imodium, among others, is a medication used to decrease the frequency of diarrhea. It is often used for this purpose in inflammatory bowel disease and short bowel syndrome. It is not recommended for those with blood in the stool, mucus in the stool, or fevers. The medication is taken by mouth.
The therapeutic index is a quantitative measurement of the relative safety of a drug. It is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes toxicity. The related terms therapeutic window or safety window refer to a range of doses which optimize between efficacy and toxicity, achieving the greatest therapeutic benefit without resulting in unacceptable side-effects or toxicity.

Adderall and Mydayis are trade names for a combination drug called mixed amphetamine salts containing four salts of amphetamine. The mixture is composed of equal parts racemic amphetamine and dextroamphetamine, which produces a (3:1) ratio between dextroamphetamine and levoamphetamine, the two enantiomers of amphetamine. Both enantiomers are stimulants, but differ enough to give Adderall an effects profile distinct from those of racemic amphetamine or dextroamphetamine, which are marketed as Evekeo and Dexedrine/Zenzedi, respectively. Adderall is used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. It is also used illicitly as an athletic performance enhancer, cognitive enhancer, appetite suppressant, and recreationally as a euphoriant. It is a central nervous system (CNS) stimulant of the phenethylamine class.
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
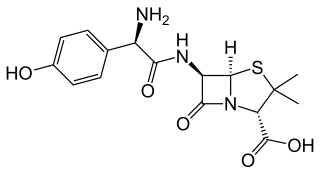
Amoxicillin/clavulanic acid, also known as co-amoxiclav or amox-clav, sold under the brand name Augmentin, among others, is an antibiotic medication used for the treatment of a number of bacterial infections. It is a combination consisting of amoxicillin, a β-lactam antibiotic, and potassium clavulanate, a β-lactamase inhibitor. It is specifically used for otitis media, streptococcal pharyngitis, pneumonia, cellulitis, urinary tract infections, and animal bites. It is taken by mouth or by injection into a vein.

In drug development, preclinical development, also termed preclinical studies or nonclinical studies, is a stage of research that begins before clinical trials and during which important feasibility, iterative testing and drug safety data are collected, typically in laboratory animals.

Selegiline, also known as L-deprenyl and sold under the brand names Eldepryl and Emsam among others, is a medication which is used in the treatment of Parkinson's disease and major depressive disorder. It is provided in the form of a capsule or tablet taken by mouth for Parkinson's disease and as a patch applied to skin for depression.

Physiologically based pharmacokinetic (PBPK) modeling is a mathematical modeling technique for predicting the absorption, distribution, metabolism and excretion (ADME) of synthetic or natural chemical substances in humans and other animal species. PBPK modeling is used in pharmaceutical research and drug development, and in health risk assessment for cosmetics or general chemicals.

Mercaptopurine (6-MP), sold under the brand name Purinethol among others, is a medication used for cancer and autoimmune diseases. Specifically it is used to treat acute lymphocytic leukemia (ALL), acute promyelocytic leukemia (APL), Crohn's disease, and ulcerative colitis. For acute lymphocytic leukemia it is generally used with methotrexate. It is taken by mouth.

Omigapil is a drug that was developed by Novartis and tested in clinical trials for its ability to help treat Parkinson's disease (PD) and amyotrophic lateral sclerosis (ALS). The development for PD and ALS have been terminated due to lack of benefit, but Santhera Pharmaceuticals bought the compound for development for the treatment of congenital muscular dystrophy (CMD).

Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade.
Pharmacokinetics, sometimes abbreviated as PK, is a branch of pharmacology dedicated to determining the fate of substances administered to a living organism. The substances of interest include any chemical xenobiotic such as: pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is the study of how an organism affects a drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models.

Lisdexamfetamine, sold under the brand name Vyvanse among others, is a stimulant medication that is mainly used to treat attention deficit hyperactivity disorder (ADHD) in people over the age of five as well as moderate-to-severe binge eating disorder in adults. Lisdexamfetamine is taken by mouth. Its effects generally begin within 2 hours and last for up to 14 hours. In the United Kingdom, it is usually less preferred than methylphenidate for the treatment of children.

Bevirimat is an anti-HIV drug derived from a betulinic acid-like compound, first isolated from Syzygium claviflorum, a Chinese herb. It is believed to inhibit HIV by a novel mechanism, so-called maturation inhibition. It is not currently U.S. Food and Drug Administration (FDA) approved. It was originally developed by the pharmaceutical company Panacos and reached Phase IIb clinical trials. Myriad Genetics announced on January 21, 2009 the acquisition of all rights to bevirimat for $7M USD. On June 8, 2010 Myriad Genetics announced that it was halting the development of maturation inhibitors, including bevirimat, to focus more on their oncology portfolio.

Deramciclane (EGIS-3886) is a non-benzodiazepine-type anxiolytic drug to treat various types of anxiety disorders. Deramciclane is a unique alternative to current anxiolytics on the market because it has a novel chemical structure and target. It acts as an antagonist at the 5-HT2A receptor, as an inverse agonist at the 5-HT2C receptor, and as a GABA reuptake inhibitor. The two serotonin receptors are G protein-coupled receptors and are two of the main excitatory serotonin receptor types. Their excitation has been implicated in anxiety and mood. Deramciclane does not affect CYP3A4 activity in metabolizing other drugs, but it is a weak inhibitor of CYP2D6. Some studies also show the drug to have moderate affinity to dopamine D2 receptors and low affinity to dopamine receptor D1. Researchers are looking for alternatives to benzodiazepines for anxiolytic use because benzodiazepine drugs have sedative and muscle relaxant side effects.
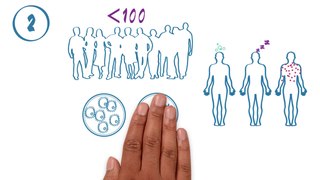
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.