
Electroporation, or electropermeabilization, is a microbiology technique in which an electrical field is applied to cells in order to increase the permeability of the cell membrane, allowing chemicals, drugs, electrode arrays or DNA to be introduced into the cell. In microbiology, the process of electroporation is often used to transform bacteria, yeast, or plant protoplasts by introducing new coding DNA. If bacteria and plasmids are mixed together, the plasmids can be transferred into the bacteria after electroporation, though depending on what is being transferred, cell-penetrating peptides or CellSqueeze could also be used. Electroporation works by passing thousands of volts across suspended cells in an electroporation cuvette. Afterwards, the cells have to be handled carefully until they have had a chance to divide, producing new cells that contain reproduced plasmids. This process is approximately ten times more effective in increasing cell membrane's permeability than chemical transformation.

A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.

Small interfering RNA (siRNA), sometimes known as short interfering RNA or silencing RNA, is a class of double-stranded RNA at first non-coding RNA molecules, typically 20-24 base pairs in length, similar to miRNA, and operating within the RNA interference (RNAi) pathway. It interferes with the expression of specific genes with complementary nucleotide sequences by degrading mRNA after transcription, preventing translation.
Transfection is the process of deliberately introducing naked or purified nucleic acids into eukaryotic cells. It may also refer to other methods and cell types, although other terms are often preferred: "transformation" is typically used to describe non-viral DNA transfer in bacteria and non-animal eukaryotic cells, including plant cells. In animal cells, transfection is the preferred term as transformation is also used to refer to progression to a cancerous state (carcinogenesis) in these cells. Transduction is often used to describe virus-mediated gene transfer into eukaryotic cells.
A DNA construct is an artificially-designed segment of DNA borne on a vector that can be used to incorporate genetic material into a target tissue or cell. A DNA construct contains a DNA insert, called a transgene, delivered via a transformation vector which allows the insert sequence to be replicated and/or expressed in the target cell. This gene can be cloned from a naturally occurring gene, or synthetically constructed. The vector can be delivered using physical, chemical or viral methods. Typically, the vectors used in DNA constructs contain an origin of replication, a multiple cloning site, and a selectable marker. Certain vectors can carry additional regulatory elements based on the expression system involved.

Cationic liposomes are spherical structures that contain positively charged lipids. Cationic liposomes can vary in size between 40 nm and 500 nm, and they can either have one lipid bilayer (monolamellar) or multiple lipid bilayers (multilamellar). The positive charge of the phospholipids allows cationic liposomes to form complexes with negatively charged nucleic acids through ionic interactions. Upon interacting with nucleic acids, cationic liposomes form clusters of aggregated vesicles. These interactions allow cationic liposomes to condense and encapsulate various therapeutic and diagnostic agents in their aqueous compartment or in their lipid bilayer. These cationic liposome-nucleic acid complexes are also referred to as lipoplexes. Due to the overall positive charge of cationic liposomes, they interact with negatively charged cell membranes more readily than classic liposomes. This positive charge can also create some issues in vivo, such as binding to plasma proteins in the bloodstream, which leads to opsonization. These issues can be reduced by optimizing the physical and chemical properties of cationic liposomes through their lipid composition. Cationic liposomes are increasingly being researched for use as delivery vectors in gene therapy due to their capability to efficiently transfect cells. A common application for cationic liposomes is cancer drug delivery.
Viral vectors are tools commonly used by molecular biologists to deliver genetic material into cells. This process can be performed inside a living organism or in cell culture. Viruses have evolved specialized molecular mechanisms to efficiently transport their genomes inside the cells they infect. Delivery of genes or other genetic material by a vector is termed transduction and the infected cells are described as transduced. Molecular biologists first harnessed this machinery in the 1970s. Paul Berg used a modified SV40 virus containing DNA from the bacteriophage λ to infect monkey kidney cells maintained in culture.

Exogenous DNA is DNA originating outside the organism of concern or study. Exogenous DNA can be found naturally in the form of partially degraded fragments left over from dead cells. These DNA fragments may then become integrated into the chromosomes of nearby bacterial cells to undergo mutagenesis. This process of altering bacteria is known as transformation. Bacteria may also undergo artificial transformation through chemical and biological processes. The introduction of exogenous DNA into eukaryotic cells is known as transfection. Exogenous DNA can also be artificially inserted into the genome, which revolutionized the process of genetic modification in animals. By microinjecting an artificial transgene into the nucleus of an animal embryo, the exogenous DNA is allowed to merge the cell's existing DNA to create a genetically modified, transgenic animal. The creation of transgenic animals also leads into the study of altering sperm cells with exogenous DNA.
A human artificial chromosome (HAC) is a microchromosome that can act as a new chromosome in a population of human cells. That is, instead of 46 chromosomes, the cell could have 47 with the 47th being very small, roughly 6–10 megabases (Mb) in size instead of 50–250 Mb for natural chromosomes, and able to carry new genes introduced by human researchers. Ideally, researchers could integrate different genes that perform a variety of functions, including disease defense.
Impalefection is a method of gene delivery using nanomaterials, such as carbon nanofibers, carbon nanotubes, nanowires. Needle-like nanostructures are synthesized perpendicular to the surface of a substrate. Plasmid DNA containing the gene, and intended for intracellular delivery, is attached to the nanostructure surface. A chip with arrays of these needles is then pressed against cells or tissue. Cells that are impaled by nanostructures can express the delivered gene(s).

Gene delivery is the process of introducing foreign genetic material, such as DNA or RNA, into host cells. Gene delivery must reach the genome of the host cell to induce gene expression. Successful gene delivery requires the foreign gene delivery to remain stable within the host cell and can either integrate into the genome or replicate independently of it. This requires foreign DNA to be synthesized as part of a vector, which is designed to enter the desired host cell and deliver the transgene to that cell's genome. Vectors utilized as the method for gene delivery can be divided into two categories, recombinant viruses and synthetic vectors.
Magnetofection is a transfection method that uses magnetic fields to concentrate particles containing vectors to target cells in the body. Magnetofection has been adapted to a variety of vectors, including nucleic acids, non-viral transfection systems, and viruses. This method offers advantages such as high transfection efficiency and biocompatibility which are balanced with limitations.

Minicircles are small (~4kb) circular replicons. They occur naturally in some eukaryotic organelle genomes. In the mitochondria-derived kinetoplast of trypanosomes, minicircles encode guide RNAs for RNA editing. In Amphidinium, the chloroplast genome is made of minicircles that encode chloroplast proteins.

Molecular cloning is a set of experimental methods in molecular biology that are used to assemble recombinant DNA molecules and to direct their replication within host organisms. The use of the word cloning refers to the fact that the method involves the replication of one molecule to produce a population of cells with identical DNA molecules. Molecular cloning generally uses DNA sequences from two different organisms: the species that is the source of the DNA to be cloned, and the species that will serve as the living host for replication of the recombinant DNA. Molecular cloning methods are central to many contemporary areas of modern biology and medicine.

Gene therapy utilizes the delivery of DNA into cells, which can be accomplished by several methods, summarized below. The two major classes of methods are those that use recombinant viruses and those that use naked DNA or DNA complexes.

Genetic engineering techniques allow the modification of animal and plant genomes. Techniques have been devised to insert, delete, and modify DNA at multiple levels, ranging from a specific base pair in a specific gene to entire genes. There are a number of steps that are followed before a genetically modified organism (GMO) is created. Genetic engineers must first choose what gene they wish to insert, modify, or delete. The gene must then be isolated and incorporated, along with other genetic elements, into a suitable vector. This vector is then used to insert the gene into the host genome, creating a transgenic or edited organism.
Gene therapy is being studied as a treatment for osteoarthritis (OA). Unlike pharmacological treatments which are administered systemically, gene therapy aims to establish sustained, synthesis of gene products and tissue rehabilitation within the joint.
Nanoinjection is the process of using a microscopic lance and electrical forces to deliver DNA to a cell. It is claimed to be more effective than microinjection because the lance used is ten times smaller than a micropipette and the method uses no fluid. The nanoinjector mechanism is operated while submerged in a pH buffered solution. Then, a positive electrical charge is applied to the lance, which accumulates negatively charged DNA on its surface. The nanoinjector mechanism then penetrates the zygotic membranes, and a negative charge is applied to the lance, releasing the accumulated DNA within the cell. The lance is required to maintain a constant elevation on both entry and exit of the cell.
Intracerebroventricular injection is an invasive injection technique of substances directly into the cerebrospinal fluid in cerebral ventricles in order to bypass the blood–brain barrier. Although this barrier effectively protects the brain, it can prevent important medications from entering the CNS. The technique is widely used in biomedical research to introduce drugs, therapeutic RNAs, plasmid DNAs, and viral vectors into the CNS of diseased mice models. It can also be used in human in cases of neurodegenerative disorders like spinal muscular atrophy (SMA), or administering chemotherapy in gliomas as well as delivering neurotrophic factors to CNS. It can be contrasted with intraperitoneal injection as an alternative choice of route of administration with differing pharmacokinetic and pharmacodynamic effects.
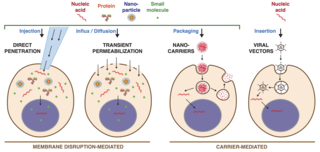
Intracellular delivery is the process of introducing external materials into living cells. Materials that are delivered into cells include nucleic acids, proteins, peptides, impermeable small molecules, synthetic nanomaterials, organelles, and micron-scale tracers, devices and objects. Such molecules and materials can be used to investigate cellular behavior, engineer cell operations or correct a pathological function.












