Related Research Articles
A growth factor is a naturally occurring substance capable of stimulating cell proliferation, wound healing, and occasionally cellular differentiation. Usually it is a secreted protein or a steroid hormone. Growth factors are important for regulating a variety of cellular processes.

Macrophages are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. This process is called phagocytosis, which acts to defend the host against infection and injury.

With physical trauma or disease suffered by an organism, healing involves the repairing of damaged tissue(s), organs and the biological system as a whole and resumption of (normal) functioning. Medicine includes the process by which the cells in the body regenerate and repair to reduce the size of a damaged or necrotic area and replace it with new living tissue. The replacement can happen in two ways: by regeneration in which the necrotic cells are replaced by new cells that form "like" tissue as was originally there; or by repair in which injured tissue is replaced with scar tissue. Most organs will heal using a mixture of both mechanisms.
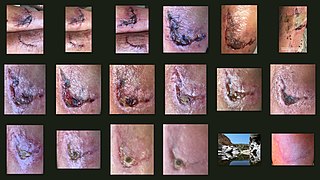
Wound healing refers to a living organism's replacement of destroyed or damaged tissue by newly produced tissue.
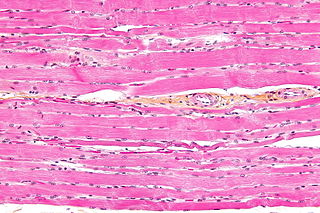
Striated muscle tissue is a muscle tissue that features repeating functional units called sarcomeres. The presence of sarcomeres manifests as a series of bands visible along the muscle fibers, which is responsible for the striated appearance observed in microscopic images of this tissue. There are two types of striated muscle:

Kupffer cells, also known as stellate macrophages and Kupffer–Browicz cells, are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. Kupffer cells comprise the largest population of tissue-resident macrophages in the body. Gut bacteria, bacterial endotoxins, and microbial debris transported to the liver from the gastrointestinal tract via the portal vein will first come in contact with Kupffer cells, the first immune cells in the liver. It is because of this that any change to Kupffer cell functions can be connected to various liver diseases such as alcoholic liver disease, viral hepatitis, intrahepatic cholestasis, steatohepatitis, activation or rejection of the liver during liver transplantation and liver fibrosis. They form part of the mononuclear phagocyte system.

Microglia are a type of neuroglia located throughout the brain and spinal cord. Microglia account for about 10-15% of cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defense in the central nervous system (CNS). Microglia originate in the yolk sac under a tightly regulated molecular process. These cells are distributed in large non-overlapping regions throughout the CNS. Microglia are key cells in overall brain maintenance—they are constantly scavenging the CNS for plaques, damaged or unnecessary neurons and synapses, and infectious agents. Since these processes must be efficient to prevent potentially fatal damage, microglia are extremely sensitive to even small pathological changes in the CNS. This sensitivity is achieved in part by the presence of unique potassium channels that respond to even small changes in extracellular potassium. Recent evidence shows that microglia are also key players in the sustainment of normal brain functions under healthy conditions. Microglia also constantly monitor neuronal functions through direct somatic contacts and exert neuroprotective effects when needed.
Stromal cells, or mesenchymal stromal cells, are differentiating cells found in abundance within bone marrow but can also be seen all around the body. Stromal cells can become connective tissue cells of any organ, for example in the uterine mucosa (endometrium), prostate, bone marrow, lymph node and the ovary. They are cells that support the function of the parenchymal cells of that organ. The most common stromal cells include fibroblasts and pericytes. The term stromal comes from Latin stromat-, "bed covering", and Ancient Greek στρῶμα, strôma, "bed".

Hepatocyte growth factor (HGF) or scatter factor (SF) is a paracrine cellular growth, motility and morphogenic factor. It is secreted by mesenchymal cells and targets and acts primarily upon epithelial cells and endothelial cells, but also acts on haemopoietic progenitor cells and T cells. It has been shown to have a major role in embryonic organ development, specifically in myogenesis, in adult organ regeneration, and in wound healing.
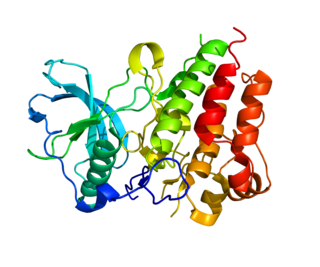
Colony stimulating factor 1 receptor (CSF1R), also known as macrophage colony-stimulating factor receptor (M-CSFR), and CD115, is a cell-surface protein encoded by the human CSF1R gene. CSF1R is a receptor that can be activated by two ligands: colony stimulating factor 1 (CSF-1) and interleukin-34 (IL-34). CSF1R is highly expressed in myeloid cells, and CSF1R signaling is necessary for the survival, proliferation, and differentiation of many myeloid cell types in vivo and in vitro. CSF1R signaling is involved in many diseases and is targeted in therapies for cancer, neurodegeneration, and inflammatory bone diseases.
A macrophage-activating factor (MAF) is a lymphokine or other receptor based signal that primes macrophages towards cytotoxicity to tumors, cytokine secretion, or clearance of pathogens. Similar molecules may cause development of an inhibitory, regulatory phenotype. A MAF can also alter the ability of macrophages to present MHC I antigen, participate in Th responses, and/or affect other immune responses.
Bone-marrow-derived macrophage (BMDM) refers to macrophage cells that are generated in a research laboratory from mammalian bone marrow cells. BMDMs can differentiate into mature macrophages in the presence of growth factors and other signaling molecules. Undifferentiated bone marrow cells are cultured in the presence of macrophage colony-stimulating factor. M-CSF is a cytokine and growth factor that is responsible for the proliferation and commitment of myeloid progenitors into monocytes. Macrophages have a wide variety of functions in the body including phagocytosis of foreign invaders and other cellular debris, releasing cytokines to trigger immune responses, and antigen presentation. BMDMs provide a large homogenous population of macrophages that play an increasingly important role in making macrophage-related research possible and financially feasible.
Tumor-associated macrophages (TAMs) are a class of immune cells present in high numbers in the microenvironment of solid tumors. They are heavily involved in cancer-related inflammation. Macrophages are known to originate from bone marrow-derived blood monocytes or yolk sac progenitors, but the exact origin of TAMs in human tumors remains to be elucidated. The composition of monocyte-derived macrophages and tissue-resident macrophages in the tumor microenvironment depends on the tumor type, stage, size, and location, thus it has been proposed that TAM identity and heterogeneity is the outcome of interactions between tumor-derived, tissue-specific, and developmental signals.
Adipose tissue macrophages (ATMs) comprise resident macrophages present in adipose tissue. Besides adipocytes, adipose tissue contains the stromal vascular fraction (SVF) of cells that includes pre-adipocytes, fibroblasts, vascular endothelial cells, and a large variety of immune cells. The latter ones are composed of mast cells, eosinophils, B cells, T cells and macrophages. The number of macrophages within adipose tissue differs depending on the metabolic status. As discovered by Rudolph Leibel and Anthony Ferrante et al. in 2003 at Columbia University, the percentage of macrophages within adipose tissue ranges from 10% in lean mice and humans up to 50% in obese leptin deficient mice, and up to 40% in obese humans. ATMs comprise nearly 50% of all immune cells in normal conditions, suggesting an important role in supporting normal functioning of the adipose tissue. Increased number of adipose tissue macrophages may correlate with increased production of pro-inflammatory molecules and might therefore contribute to the pathophysiological consequences of obesity, although is becoming recognized that in healthy conditions tissue-resident macrophages actively support a variety of critical physiological functions in nearly all organs and tissues, including adipose tissue.
Regulatory macrophages (Mregs) represent a subset of anti-inflammatory macrophages. In general, macrophages are a very dynamic and plastic cell type and can be divided into two main groups: classically activated macrophages (M1) and alternatively activated macrophages (M2). M2 group can further be divided into sub-groups M2a, M2b, M2c, and M2d. Typically the M2 cells have anti-inflammatory and regulatory properties and produce many different anti-inflammatory cytokines such as IL-4, IL-33, IL-10, IL-1RA, and TGF-β. M2 cells can also secrete angiogenic and chemotactic factors. These cells can be distinguished based on the different expression levels of various surface proteins and the secretion of different effector molecules.
Macrophage polarization is a process by which macrophages adopt different functional programs in response to the signals from their microenvironment. This ability is connected to their multiple roles in the organism: they are powerful effector cells of the innate immune system, but also important in removal of cellular debris, embryonic development and tissue repair.
Scar free healing is the process by which significant injuries can heal without permanent damage to the tissue the injury has affected. In most healing, scars form due to the fibrosis and wound contraction, however in scar free healing, tissue is completely regenerated. During the 1990s, published research on the subject increased; it is a relatively recent term in the literature. Scar free healing occurs in foetal life but the ability progressively diminishes into adulthood. In other animals such as amphibians, however, tissue regeneration occurs, for example as skin regeneration in the adult axolotl.
Craniofacial regeneration refers to the biological process by which the skull and face regrow to heal an injury. This page covers birth defects and injuries related to the craniofacial region, the mechanisms behind the regeneration, the medical application of these processes, and the scientific research conducted on this specific regeneration. This regeneration is not to be confused with tooth regeneration. Craniofacial regrowth is broadly related to the mechanisms of general bone healing.
Dedifferentiation is a transient process by which cells become less specialized and return to an earlier cell state within the same lineage. This suggests an increase in cell potency, meaning that, following dedifferentiation, a cell may possess the ability to re-differentiate into more cell types than it did before dedifferentiation. This is in contrast to differentiation, where differences in gene expression, morphology, or physiology arise in a cell, making its function increasingly specialized.
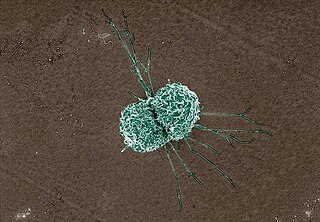
Dermal macrophages are macrophages in the skin that facilitate skin homeostasis by mediating wound repair, hair growth, and salt balance. Their functional role in these processes is the mediator of inflammation. They can acquire an M1 or M2 phenotype to promote or suppress an inflammatory response, thereby influencing other cells' activity via the production of pro-inflammatory or anti-inflammatory cytokines. Dermal macrophages' ability to acquire pro-inflammatory properties also potentiates them in cancer defence. M1 macrophages can suppress tumour growth in the skin by their pro-inflammatory properties. However, M2 macrophages support tumour growth and invasion by the production of Th2 cytokines such as TGFβ and IL-10. Thus, the exact contribution of each phenotype to cancer defence and the skin's homeostasis is still unclear.
References
- ↑ Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. (2018). "The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes". Frontiers in Physiology. 9: 419. doi: 10.3389/fphys.2018.00419 . PMC 5938667 . PMID 29765329.
- ↑ Ferrante, Christopher J.; Leibovich, Samuel Joseph (February 2012). "Regulation of Macrophage Polarization and Wound Healing". Advances in Wound Care. 1 (1): 10–16. doi:10.1089/wound.2011.0307. PMC 3623587 . PMID 24527272.
- ↑ Kotwal, Girish J.; Chien, Sufan (2017). "Macrophage Differentiation in Normal and Accelerated Wound Healing". Macrophages. Results and Problems in Cell Differentiation. Vol. 62. pp. 353–364. doi:10.1007/978-3-319-54090-0_14. ISBN 978-3-319-54089-4. PMC 5841920 . PMID 28455716.
- ↑ Schiaffino, Stefano; Pereira, Marcelo G.; Ciciliot, Stefano; Rovere-Querini, Patrizia (February 2017). "Regulatory T cells and skeletal muscle regeneration". The FEBS Journal. 284 (4): 517–524. doi: 10.1111/febs.13827 . PMID 27479876. S2CID 19730876.
- ↑ Boulter, Luke; Govaere, Olivier; Bird, Tom G.; Radulescu, Sorina; Ramachandran, Prakash; Pellicoro, Antonella; Ridgway, Rachel A.; Seo, Sang Soo; Spee, Bart; Van Rooijen, Nico; Sansom, Owen J.; Iredale, John P.; Lowell, Sally; Roskams, Tania; Forbes, Stuart J. (4 March 2012). "Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease". Nature Medicine. 18 (4): 572–579. doi:10.1038/nm.2667. PMC 3364717 . PMID 22388089.
- ↑ Kyritsis, Nikos; Kizil, Caghan; Zocher, Sara; Kroehne, Volker; Kaslin, Jan; Freudenreich, Dorian; Iltzsche, Anne; Brand, Michael (7 December 2012). "Acute inflammation initiates the regenerative response in the adult zebrafish brain". Science. 338 (6112): 1353–1356. Bibcode:2012Sci...338.1353K. doi:10.1126/science.1228773. PMID 23138980. S2CID 12295180.
- ↑ Kurimoto, Takuji; Yin, Yuqin; Habboub, Ghaith; Gilbert, Hui-Ya; Li, Yiqing; Nakao, Shintaro; Hafezi-Moghadam, Ali; Benowitz, Larry I. (11 September 2013). "Neutrophils express oncomodulin and promote optic nerve regeneration". Journal of Neuroscience. 33 (37): 14816–14824. doi: 10.1523/JNEUROSCI.5511-12.2013 . PMC 3771038 . PMID 24027282. S2CID 14640685.
- ↑ Aurora, Arin B.; Olson, Eric N. (3 July 2014). "Immune modulation of stem cells and regeneration". Cell Stem Cell. 15 (1): 14–25. doi: 10.1016/j.stem.2014.06.009 . PMC 4131296 . PMID 24996166.
- ↑ Kubin, Thomas; Pöling, Jochen; Kostin, Sawa; Gajawada, Praveen; Hein, Stefan; Rees, Wolfgang; Wietelmann, Astrid; Tanaka, Minoru; Lörchner, Holger; Schimanski, Silvia; Szibor, Marten; Warnecke, Henning; Braun, Thomas (4 November 2011). "Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling". Cell Stem Cell. 9 (5): 420–432. doi: 10.1016/j.stem.2011.08.013 . PMID 22056139.
- ↑ Lilla, Jennifer N.; Werb, Zena (1 January 2010). "Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis". Developmental Biology. 337 (1): 124–133. doi:10.1016/j.ydbio.2009.10.021. PMC 2787992 . PMID 19850030.
- ↑ Stefater, James A.; Lewkowich, Ian; Rao, Sujata; Mariggi, Giovanni; Carpenter, April C.; Burr, Adam R.; Fan, Jieqing; Ajima, Rieko; Molkentin, Jeffery D.; Williams, Bart O.; Wills-Karp, Marsha; Pollard, Jeffrey W.; Yamaguchi, Terry; Ferrara, Napoleone; Gerhardt, Holger; Lang, Richard A. (29 May 2011). "Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells". Nature. 474 (7352): 511–515. doi:10.1038/nature10085. PMC 3214992 . PMID 21623369.
- ↑ Nahrendorf, Matthias; Swirski, Filip K.; Aikawa, Elena; Stangenberg, Lars; Wurdinger, Thomas; Figueiredo, Jose-Luiz; Libby, Peter; Weissleder, Ralph; Pittet, Mikael J. (26 November 2007). "The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions". The Journal of Experimental Medicine. 204 (12): 3037–3047. doi: 10.1084/jem.20070885 . PMC 2118517 . PMID 18025128. S2CID 6666039.
- ↑ Aurora, Arin B.; Olson, Eric N. (3 July 2014). "Immune modulation of stem cells and regeneration". Cell Stem Cell. 15 (1): 14–25. doi: 10.1016/j.stem.2014.06.009 . PMC 4131296 . PMID 24996166.