Related Research Articles

Coronary artery disease (CAD), also called coronary heart disease (CHD), ischemic heart disease (IHD), myocardial ischemia, or simply heart disease, involves the reduction of blood flow to the heart muscle due to build-up of atherosclerotic plaque in the arteries of the heart. It is the most common of the cardiovascular diseases. Types include stable angina, unstable angina, and myocardial infarction. A common symptom is chest pain or discomfort which may travel into the shoulder, arm, back, neck, or jaw. Occasionally it may feel like heartburn. Usually symptoms occur with exercise or emotional stress, last less than a few minutes, and improve with rest. Shortness of breath may also occur and sometimes no symptoms are present. In many cases, the first sign is a heart attack. Other complications include heart failure or an abnormal heartbeat.

A randomized controlled trial is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments.

Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.
Selection bias is the bias introduced by the selection of individuals, groups, or data for analysis in such a way that proper randomization is not achieved, thereby failing to ensure that the sample obtained is representative of the population intended to be analyzed. It is sometimes referred to as the selection effect. The phrase "selection bias" most often refers to the distortion of a statistical analysis, resulting from the method of collecting samples. If the selection bias is not taken into account, then some conclusions of the study may be false.
A cohort study is a particular form of longitudinal study that samples a cohort, performing a cross-section at intervals through time. It is a type of panel study where the individuals in the panel share a common characteristic.

Cardiovascular disease (CVD) is any disease involving the heart or blood vessels. CVDs constitute a class of diseases that includes: coronary artery diseases, heart failure, hypertensive heart disease, rheumatic heart disease, cardiomyopathy, arrhythmia, congenital heart disease, valvular heart disease, carditis, aortic aneurysms, peripheral artery disease, thromboembolic disease, and venous thrombosis.
Dyslipidemia is a metabolic disorder characterized by abnormally high or low amounts of any or all lipids or lipoproteins in the blood. Dyslipidemia is a risk factor for the development of atherosclerotic cardiovascular diseases (ASCVD), which include coronary artery disease, cerebrovascular disease, and peripheral artery disease. Although dyslipidemia is a risk factor for ASCVD, abnormal levels don't mean that lipid lowering agents need to be started. Other factors, such as comorbid conditions and lifestyle in addition to dyslipidemia, is considered in a cardiovascular risk assessment. In developed countries, most dyslipidemias are hyperlipidemias; that is, an elevation of lipids in the blood. This is often due to diet and lifestyle. Prolonged elevation of insulin resistance can also lead to dyslipidemia. Likewise, increased levels of O-GlcNAc transferase (OGT) may cause dyslipidemia.
In clinical trials, a surrogate endpoint is a measure of effect of a specific treatment that may correlate with a real clinical endpoint but does not necessarily have a guaranteed relationship. The National Institutes of Health (USA) defines surrogate endpoint as "a biomarker intended to substitute for a clinical endpoint".

The Women's Health Initiative (WHI) was a series of clinical studies initiated by the U.S. National Institutes of Health (NIH) in 1991, to address major health issues causing morbidity and mortality in postmenopausal women. It consisted of three clinical trials (CT) and an observational study (OS). In particular, randomized controlled trials were designed and funded that addressed cardiovascular disease, cancer, and osteoporosis.
Clinical endpoints or clinical outcomes are outcome measures referring to occurrence of disease, symptom, sign or laboratory abnormality constituting a target outcome in clinical research trials. The term may also refer to any disease or sign that strongly motivates withdrawal of an individual or entity from the trial, then often termed a humane (clinical) endpoint.

In causal inference, a confounder is a variable that influences both the dependent variable and independent variable, causing a spurious association. Confounding is a causal concept, and as such, cannot be described in terms of correlations or associations. The existence of confounders is an important quantitative explanation why correlation does not imply causation. Some notations are explicitly designed to identify the existence, possible existence, or non-existence of confounders in causal relationships between elements of a system.

Percutaneous coronary intervention (PCI) is a minimally invasive non-surgical procedure used to treat narrowing of the coronary arteries of the heart found in coronary artery disease. The procedure is used to place and deploy coronary stents, a permanent wire-meshed tube, to open narrowed coronary arteries. PCI is considered 'non-surgical' as it uses a small hole in a peripheral artery (leg/arm) to gain access to the arterial system, an equivalent surgical procedure would involve the opening of the chest wall to gain access to the heart area. The term 'coronary angioplasty with stent' is synonymous with PCI. The procedure visualises the blood vessels via fluoroscopic imaging and contrast dyes. PCI is performed by an interventional cardiologists in a catheterization laboratory setting.
A hierarchy of evidence, comprising levels of evidence (LOEs), that is, evidence levels (ELs), is a heuristic used to rank the relative strength of results obtained from experimental research, especially medical research. There is broad agreement on the relative strength of large-scale, epidemiological studies. More than 80 different hierarchies have been proposed for assessing medical evidence. The design of the study and the endpoints measured affect the strength of the evidence. In clinical research, the best evidence for treatment efficacy is mainly from meta-analyses of randomized controlled trials (RCTs). Systematic reviews of completed, high-quality randomized controlled trials – such as those published by the Cochrane Collaboration – rank the same as systematic review of completed high-quality observational studies in regard to the study of side effects. Evidence hierarchies are often applied in evidence-based practices and are integral to evidence-based medicine (EBM).

In fields such as epidemiology, social sciences, psychology and statistics, an observational study draws inferences from a sample to a population where the independent variable is not under the control of the researcher because of ethical concerns or logistical constraints. One common observational study is about the possible effect of a treatment on subjects, where the assignment of subjects into a treated group versus a control group is outside the control of the investigator. This is in contrast with experiments, such as randomized controlled trials, where each subject is randomly assigned to a treated group or a control group. Observational studies, for lacking an assignment mechanism, naturally present difficulties for inferential analysis.
A glossary of terms used in clinical research.
The following outline is provided as an overview of and topical guide to clinical research:
HeartScore is a cardiovascular disease risk assessment and management tool developed by the European Society of Cardiology, aimed at supporting clinicians in optimising individual cardiovascular risk reduction.
The Framingham Risk Score is a sex-specific algorithm used to estimate the 10-year cardiovascular risk of an individual. The Framingham Risk Score was first developed based on data obtained from the Framingham Heart Study, to estimate the 10-year risk of developing coronary heart disease. In order to assess the 10-year cardiovascular disease risk, cerebrovascular events, peripheral artery disease and heart failure were subsequently added as disease outcomes for the 2008 Framingham Risk Score, on top of coronary heart disease.

Gender-biased diagnosing is the idea that medical and psychological diagnosis are influenced by the gender of the patient. Several studies have found evidence of differential diagnosis for patients with similar ailments but of different sexes. Female patients face discrimination through the denial of treatment or miss-classification of diagnosis as a result of not being taken seriously due to stereotypes and gender bias. According to traditional medical studies, most of these medical studies were done on men thus overlooking many issues that were related to women's health. This topic alone sparked controversy and brought about question to the medical standard of our time. Popular media has illuminated the issue of gender bias in recent years. Research that was done on diseases that affected women more were less funded than those diseases that affected men and women equally.
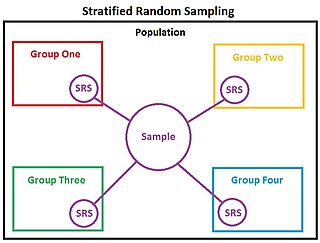
In statistics, stratified randomization is a method of sampling which first stratifies the whole study population into subgroups with same attributes or characteristics, known as strata, then followed by simple random sampling from the stratified groups, where each element within the same subgroup are selected unbiasedly during any stage of the sampling process, randomly and entirely by chance. Stratified randomization is considered a subdivision of stratified sampling, and should be adopted when shared attributes exist partially and vary widely between subgroups of the investigated population, so that they require special considerations or clear distinctions during sampling. This sampling method should be distinguished from cluster sampling, where a simple random sample of several entire clusters is selected to represent the whole population, or stratified systematic sampling, where a systematic sampling is carried out after the stratification process. Stratified random sampling is sometimes also known as "quota random sampling".
References
- 1 2 3 Van Spall, Harriette (21 March 2007). "Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review". The Journal of the American Medical Association. 297 (11): 1233–40. doi:10.1001/jama.297.11.1233. PMID 17374817.
- ↑ Helfand M, Buckley D, Fleming C, et al. (2009). Screening for Intermediate Risk Factors for Coronary Heart Disease. Rockville (MD): Agency for Healthcare Research and Quality (US).
- ↑ Pittman, Corinne A.; Roura, Raúl; Price, Carrie; Lin, Frank R.; Marrone, Nicole; Nieman, Carrie L. (2021-07-01). "Racial/Ethnic and Sex Representation in US-Based Clinical Trials of Hearing Loss Management in Adults: A Systematic Review". JAMA Otolaryngology–Head & Neck Surgery. 147 (7): 656. doi:10.1001/jamaoto.2021.0550. ISSN 2168-6181.