Related Research Articles
Evidence-based medicine (EBM) is "the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients." The aim of EBM is to integrate the experience of the clinician, the values of the patient, and the best available scientific information to guide decision-making about clinical management. The term was originally used to describe an approach to teaching the practice of medicine and improving decisions by individual physicians about individual patients.
A meta-analysis is a statistical analysis that combines the results of multiple scientific studies. Meta-analyses can be performed when there are multiple scientific studies addressing the same question, with each individual study reporting measurements that are expected to have some degree of error. The aim then is to use approaches from statistics to derive a pooled estimate closest to the unknown common truth based on how this error is perceived. It is thus a basic methodology of metascience. Meta-analytic results are considered the most trustworthy source of evidence by the evidence-based medicine literature.

A placebo can be roughly defined as a sham medical treatment. Common placebos include inert tablets, inert injections, sham surgery, and other procedures.

A randomized controlled trial is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments.

The Cochrane Collaboration is a British international charitable organisation formed to synthesise medical research findings to facilitate evidence-based choices about health interventions involving health professionals, patients and policy makers. It includes 53 review groups that are based at research institutions worldwide. Cochrane has approximately 30,000 volunteer experts from around the world.
In a blind or blinded experiment, information which may influence the participants of the experiment is withheld until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, observer's effect on the participants, observer bias, confirmation bias, and other sources. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. In some cases, while blinding would be useful, it is impossible or unethical. For example, it is not possible to blind a patient to their treatment in a physical therapy intervention. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constraints.
In published academic research, publication bias occurs when the outcome of an experiment or research study biases the decision to publish or otherwise distribute it. Publishing only results that show a significant finding disturbs the balance of findings in favor of positive results. The study of publication bias is an important topic in metascience.

A systematic review is a scholarly synthesis of the evidence on a clearly presented topic using critical methods to identify, define and assess research on the topic. A systematic review extracts and interprets data from published studies on the topic, then analyzes, describes, critically appraises and summarizes interpretations into a refined evidence-based conclusion. For example, a systematic review of randomized controlled trials is a way of summarizing and implementing evidence-based medicine.
In statistics, (between-) study heterogeneity is a phenomenon that commonly occurs when attempting to undertake a meta-analysis. In a simplistic scenario, studies whose results are to be combined in the meta-analysis would all be undertaken in the same way and to the same experimental protocols. Differences between outcomes would only be due to measurement error. Study heterogeneity denotes the variability in outcomes that goes beyond what would be expected due to measurement error alone.
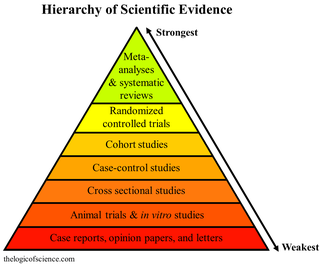
A hierarchy of evidence, comprising levels of evidence (LOEs), that is, evidence levels (ELs), is a heuristic used to rank the relative strength of results obtained from experimental research, especially medical research. There is broad agreement on the relative strength of large-scale, epidemiological studies. More than 80 different hierarchies have been proposed for assessing medical evidence. The design of the study and the endpoints measured affect the strength of the evidence. In clinical research, the best evidence for treatment efficacy is mainly from meta-analyses of randomized controlled trials (RCTs). Systematic reviews of completed, high-quality randomized controlled trials – such as those published by the Cochrane Collaboration – rank the same as systematic review of completed high-quality observational studies in regard to the study of side effects. Evidence hierarchies are often applied in evidence-based practices and are integral to evidence-based medicine (EBM).
Sir Rory Edwards Collins FMedSci FRS is a British physician who is Professor of Medicine and Epidemiology at the Clinical Trial Service Unit within the University of Oxford, the head of the Nuffield Department of Population Health and a Fellow of Green Templeton College, Oxford. His work has been in the establishment of large-scale epidemiological studies of the causes, prevention and treatment of heart attacks, other vascular disease, and cancer, while also being closely involved in developing approaches to the combination of results from related studies ("meta-analyses"). Since September 2005, he has been the Principal Investigator and Chief Executive of the UK Biobank, a prospective study of 500,000 British people aged 40–69 at recruitment.
Peter Christian Gøtzsche is a Danish physician, medical researcher, and former leader of the Nordic Cochrane Center at Rigshospitalet in Copenhagen, Denmark. He is a co-founder of the Cochrane Collaboration and has written numerous reviews for the organization. His membership in Cochrane was terminated by its Governing Board of Trustees on 25 September 2018.

Preventable causes of death are causes of death related to risk factors which could have been avoided. The World Health Organization has traditionally classified death according to the primary type of disease or injury. However, causes of death may also be classified in terms of preventable risk factors—such as smoking, unhealthy diet, sexual behavior, and reckless driving—which contribute to a number of different diseases. Such risk factors are usually not recorded directly on death certificates, although they are acknowledged in medical reports.

John P. A. Ioannidis is a Greek-American physician-scientist, writer and Stanford University professor who has made contributions to evidence-based medicine, epidemiology, and clinical research. Ioannidis studies scientific research itself, meta-research primarily in clinical medicine and the social sciences.

PRISMA is an evidence-based minimum set of items aimed at helping scientific authors to report a wide array of systematic reviews and meta-analyses, primarily used to assess the benefits and harms of a health care intervention. PRISMA focuses on ways in which authors can ensure a transparent and complete reporting of this type of research. The PRISMA standard superseded the earlier QUOROM standard. It offers the replicability of a systematic literature review. Researchers have to figure out research objectives that answer the research question, states the keywords, a set of exclusion and inclusion criteria. In the review stage, relevant articles were searched, irrelevant ones are removed. Articles are analyzed according to some pre-defined categories.
Alessandro Liberati was an Italian healthcare researcher and clinical epidemiologist, and founder of the Italian Cochrane Centre.

AllTrials is a project advocating that clinical research adopt the principles of open research. The project summarizes itself as "All trials registered, all results reported": that is, all clinical trials should be listed in a clinical trials registry, and their results should always be shared as open data.
Lesley Ann Stewart is a Scottish academic whose research interests are in the development and application of evidence synthesis methods, particularly systematic reviews and individual participant data meta-analysis. She is head of department for the Centre for Reviews and Dissemination at the University of York and director for the NIHR Evidence Synthesis Programme. She was one of the founders of the Cochrane Collaboration in 1993. Stewart served as president of the Society for Research Synthesis Methodology (2013-2016) and was a founding co-editor in chief of the academic journal Systematic Reviews (2010–2021).
Allegiance bias in behavioral sciences is a bias resulted from the investigator's or researcher's allegiance to a specific school of thought. Researchers/investigators have been exposed to many types of branches of psychology or schools of thought. Naturally they adopt a school or branch that fits with their paradigm of thinking. More specifically, allegiance bias is when this leads therapists, researchers, etc. believing that their school of thought or treatment is superior to others. Their superior belief to these certain schools of thought can bias their research in effective treatments trials or investigative situations leading to allegiance bias. Reason being is that they may have devoted their thinking to certain treatments they have seen work in their past experiences. This can lead to errors in interpreting the results of their research. Their “pledge” to stay within their own paradigm of thinking may affect their ability to find more effective treatments to help the patient or situation they are investigating.
Azeem Majeed is a Professor and Head of the Department of Primary Care & Public Health at Imperial College, London, as well as a general practitioner in South London and a consultant in public health. In the most recent UK University Research Excellence Framework results, Imperial College London was the highest ranked university in the UK for the quality of research in the “Public Health, Health Services and Primary Care” unit of assessment.
References
- ↑ Taichman, Darren B.; Backus, Joyce; Baethge, Christopher; Bauchner, Howard; de Leeuw, Peter W.; Drazen, Jeffrey M.; Fletcher, John; Frizelle, Frank A.; Groves, Trish; Haileamlak, Abraham; James, Astrid; Laine, Christine; Peiperl, Larry; Pinborg, Anja; Sahni, Peush; Wu, Sinan (2016). "Sharing Clinical Trial Data: A Proposal From the International Committee of Medical Journal Editors". Annals of Internal Medicine. 164 (7): 127–128. doi:10.7326/M15-2928. ISSN 0003-4819. PMC 4799536 . PMID 26830980.
- ↑ Tierney, Jayne F.; Pignon, Jean-Pierre; Gueffyier, Francois; Clarke, Mike; Askie, Lisa; Vale, Claire L.; Burdett, Sarah (November 2015). "How individual participant data meta-analyses have influenced trial design, conduct, and analysis". Journal of Clinical Epidemiology. 68 (11): 1325–1335. doi:10.1016/j.jclinepi.2015.05.024. PMC 4635379 . PMID 26186982.
- ↑ Thomas, Doneal; Radji, Sanyath; Benedetti, Andrea (19 June 2014). "Systematic review of methods for individual patient data meta- analysis with binary outcomes". BMC Medical Research Methodology. 14 (1): 79. doi: 10.1186/1471-2288-14-79 . PMC 4074845 . PMID 24943877.
- ↑ Tierney, Jayne F.; Vale, Claire; Riley, Richard; Smith, Catrin Tudur; Stewart, Lesley; Clarke, Mike; Rovers, Maroeska (2015-07-21). "Individual Participant Data (IPD) Meta-analyses of Randomised Controlled Trials: Guidance on Their Use". PLOS Medicine. 12 (7): e1001855. doi: 10.1371/journal.pmed.1001855 . ISSN 1549-1676. PMC 4510878 . PMID 26196287.
- 1 2 3 Debray, Thomas P. A.; Riley, Richard D.; Rovers, Maroeska M.; Reitsma, Johannes B.; Moons, Karel G. M.; Group, Cochrane IPD Meta-analysis Methods (2015-10-13). "Individual Participant Data (IPD) Meta-analyses of Diagnostic and Prognostic Modeling Studies: Guidance on Their Use". PLOS Medicine. 12 (10): e1001886. doi: 10.1371/journal.pmed.1001886 . ISSN 1549-1676. PMC 4603958 . PMID 26461078.
- ↑ Riley, R. D.; Lambert, P. C.; Abo-Zaid, G. (5 February 2010). "Meta-analysis of individual participant data: rationale, conduct, and reporting". BMJ. 340 (feb05 1): c221. doi: 10.1136/bmj.c221 . PMID 20139215.