Related Research Articles

An artificial cardiac pacemaker is a medical device, nowadays always implanted, that generates electrical pulses delivered by electrodes to one or more of the chambers of the heart, the upper atria or lower ventricles. Each pulse causes the targeted chamber(s) to contract and pump blood, thus regulating the function of the electrical conduction system of the heart.

Defibrillation is a treatment for life-threatening cardiac arrhythmias, specifically ventricular fibrillation (V-Fib) and non-perfusing ventricular tachycardia (V-Tach). A defibrillator delivers a dose of electric current to the heart. Although not fully understood, this process depolarizes a large amount of the heart muscle, ending the arrhythmia. Subsequently, the body's natural pacemaker in the sinoatrial node of the heart is able to re-establish normal sinus rhythm. A heart which is in asystole (flatline) cannot be restarted by a defibrillator; it would be treated only by cardiopulmonary resuscitation (CPR) and medication, and then by cardioversion or defibrillation if it converts into a shockable rhythm.

An implantable cardioverter-defibrillator (ICD) or automated implantable cardioverter defibrillator (AICD) is a device implantable inside the body, able to perform defibrillation, and depending on the type, cardioversion and pacing of the heart. The ICD is the first-line treatment and prophylactic therapy for patients at risk for sudden cardiac death due to ventricular fibrillation and ventricular tachycardia.
Multicenter Automatic Defibrillator Implantation Trial and MADIT II are implantable cardioverter defibrillator trials which investigate whether prophylactic ICD therapy in moderately high-risk coronary patients would significantly reduce death compared with patients treated with conventional therapy alone.
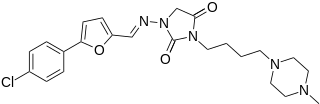
Azimilide is a class ΙΙΙ antiarrhythmic drug. The agents from this heterogeneous group have an effect on the repolarization, they prolong the duration of the action potential and the refractory period. Also they slow down the spontaneous discharge frequency of automatic pacemakers by depressing the slope of diastolic depolarization. They shift the threshold towards zero or hyperpolarize the membrane potential. Although each agent has its own properties and will have thus a different function.

T wave alternans (TWA) is a periodic beat-to-beat variation in the amplitude or shape of the T wave in an electrocardiogram TWA was first described in 1908. At that time, only large variations could be detected. Those large TWAs were associated with increased susceptibility to lethal ventricular tachycardias.
In statistics, sequential analysis or sequential hypothesis testing is statistical analysis where the sample size is not fixed in advance. Instead data are evaluated as they are collected, and further sampling is stopped in accordance with a pre-defined stopping rule as soon as significant results are observed. Thus a conclusion may sometimes be reached at a much earlier stage than would be possible with more classical hypothesis testing or estimation, at consequently lower financial and/or human cost.

MedCalc is a statistical software package designed for the biomedical sciences. It has an integrated spreadsheet for data input and can import files in several formats.
Repeated measures design is a research design that involves multiple measures of the same variable taken on the same or matched subjects either under different conditions or over two or more time periods. For instance, repeated measurements are collected in a longitudinal study in which change over time is assessed.
The Newman–Keuls or Student–Newman–Keuls (SNK)method is a stepwise multiple comparisons procedure used to identify sample means that are significantly different from each other. It was named after Student (1927), D. Newman, and M. Keuls. This procedure is often used as a post-hoc test whenever a significant difference between three or more sample means has been revealed by an analysis of variance (ANOVA). The Newman–Keuls method is similar to Tukey's range test as both procedures use studentized range statistics. Unlike Tukey's range test, the Newman–Keuls method uses different critical values for different pairs of mean comparisons. Thus, the procedure is more likely to reveal significant differences between group means and to commit type I errors by incorrectly rejecting a null hypothesis when it is true. In other words, the Neuman-Keuls procedure is more powerful but less conservative than Tukey's range test.
A wearable cardioverter defibrillator (WCD) is a non-invasive, external device for patients at risk of sudden cardiac arrest (SCA). It allows physicians time to assess their patient's arrhythmic risk and see if their ejection fraction improves before determining the next steps in patient care. It is a leased device. A summary of the device, its technology and indications was published in 2017 and reviewed by the EHRA Scientific Documents Committee.
Cameron Health was a medical device developer based in San Clemente, California, USA. Cameron Health had its European office, Cameron Health BV, in Arnhem, The Netherlands. The privately held company's focus was on a new generation of minimally invasive implantable cardioverter-defibrillator (ICD) which they called a Subcutaneous Implantable Defibrillator (S-ICD). Cameron Health's approach avoided implanting transvenous leads into the heart, which had been the usual procedure for cardiac devices. Instead, the Cameron ICD was entirely implanted outside the thoracic wall.
The Haybittle–Peto boundary is a rule for deciding when to stop a clinical trial prematurely. It is named for John Haybittle and Richard Peto.
The Pocock boundary is a method for determining whether to stop a clinical trial prematurely. The typical clinical trial compares two groups of patients. One group are given a placebo or conventional treatment, while the other group of patients are given the treatment that is being tested. The investigators running the clinical trial will wish to stop the trial early for ethical reasons if the treatment group clearly shows evidence of benefit. In other words, "when early results proved so promising it was no longer fair to keep patients on the older drugs for comparison, without giving them the opportunity to change."
Yaariv Khaykin is a Canadian cardiologist and a clinical researcher in the area of electrophysiology. He is the director of the Newmarket Electrophysiology Research Group at the Southlake Regional Health Centre. He has published research into complex ablation and pioneered cardiac ablation methods.

The growth curve model in statistics is a specific multivariate linear model, also known as GMANOVA. It generalizes MANOVA by allowing post-matrices, as seen in the definition.

Subcutaneous implantable cardioverter defibrillator, or S-ICD, is an implantable medical device for detecting and terminating ventricular tachycardia and ventricular fibrillation in patients at risk of sudden cardiac arrest. It is a type of implantable cardioverter defibrillator but unlike the transvenous ICD, the S-ICD lead is placed just under the skin, leaving the heart and veins untouched.
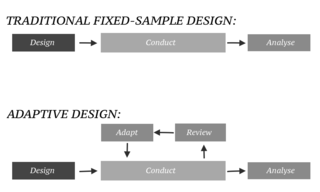
In an adaptive design of a clinical trial, the parameters and conduct of the trial for a candidate drug or vaccine may be changed based on an interim analysis. Adaptive design typically involves advanced statistics to interpret a clinical trial endpoint. This is in contrast to traditional single-arm clinical trials or randomized clinical trials (RCTs) that are static in their protocol and do not modify any parameters until the trial is completed. The adaptation process takes place at certain points in the trial, prescribed in the trial protocol. Importantly, this trial protocol is set before the trial begins with the adaptation schedule and processes specified. Adaptions may include modifications to: dosage, sample size, drug undergoing trial, patient selection criteria and/or "cocktail" mix. The PANDA provides not only a summary of different adaptive designs, but also comprehensive information on adaptive design planning, conduct, analysis and reporting.
Ajit C. Tamhane is a professor in the Department of Industrial Engineering and Management Sciences (IEMS) at Northwestern University and also holds a courtesy appointment in the Department of Statistics.
References
- ↑ Armitage, P.; McPherson, C.K.; Rowe, B.C. (1969). "Repeated Significance Tests on Accumulating Data". Journal of the Royal Statistical Society, Series A. 132 (2): 235–244. doi:10.2307/2343787. JSTOR 2343787.
- ↑ McPherson, C.K.; Armitage, P. (1971). "Repeated Significance Tests on Accumulating Data". Journal of the Royal Statistical Society, Series A. 134 (1): 15–26. doi:10.2307/2343971. JSTOR 2343971.
- ↑ Pocock, S.J. (1977). "Group sequential methods in the design and analysis of clinical trials". Biometrika . 64 (2): 191–199. doi:10.2307/2335684. JSTOR 2335684.
- ↑ Pocock, S.J. (1982). "Interim Analyses for Randomized Clinical trials: The Group Sequential Approach". Biometrics . 38 (1): 153–162. doi:10.2307/2530298. JSTOR 2530298. PMID 7082757.
- ↑ O’Brien, P.C.; Fleming, T.R. (1979). "A Multiple Testing Procedure for Clinical Trials". Biometrics. 35 (3): 549–556. doi:10.2307/2530245. JSTOR 2530245. PMID 497341.
- ↑ Lan, K.G.; DeMets, D.L. (1983). "Discrete sequential boundaries for clinical trials". Biometrika. 70 (3): 659–663. doi:10.1093/biomet/70.3.659. JSTOR 2336502.
- ↑ Tang, Z (2015). "Optimal futility interim design: a predictive probability of success approach with time to event end point". Journal of Biopharmaceutical Statistics. 25 (6): 1312–1319. doi:10.1080/10543406.2014.983646. PMID 25379701. S2CID 1375275.
- ↑ Tang, Z (2017). "Defensive Efficacy Interim Design: dynamic benefit/risk ratio view using probability of success". Journal of Biopharmaceutical Statistics, 2016. 27 (4): 683–690. doi:10.1080/10543406.2016.1198370. PMID 27295497. S2CID 46723221.
- ↑ Jennison, Christopher; Turnbull, Bruce C. (1999). Group Sequential Methods with Applications to Clinical Trials. Boca Raton, Florida: Chapman & Hall/CRC. ISBN 978-0-8493-0316-6.
- ↑ Chin, Richard (2012). Adaptive and Flexible Clinical Trials. Boca Raton, Florida: Chapman & Hall/CRC. ISBN 978-1-4398-3832-7.
- ↑ Chow, Shein-Chow; Chang, Mark (2012). Adaptive Design Methods in Clinical Trials (2 ed.). Boca Raton, Florida: Chapman & Hall/CRC. ISBN 978-1-4398-3987-4.
- ↑ Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML (2002). "Prophylactic Implantation of a Defibrillator in Patients with Myocardial Infarction and Reduced Ejection Fraction". New England Journal of Medicine. 346 (12): 877–83. doi: 10.1056/NEJMoa013474 . PMID 11907286.