Related Research Articles

Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

Health care, or healthcare, is the improvement of health via the prevention, diagnosis, treatment, amelioration or cure of disease, illness, injury, and other physical and mental impairments in people. Health care is delivered by health professionals and allied health fields. Medicine, dentistry, pharmacy, midwifery, nursing, optometry, audiology, psychology, occupational therapy, physical therapy, athletic training, and other health professions all constitute health care. The term includes work done in providing primary care, secondary care, and tertiary care, as well as in public health.

.eu is the country code top-level domain (ccTLD) for the European Union (EU). Launched on 7 December 2005, the domain is available for any person, company or organization based in the European Union. This was extended to the European Economic Area in 2014, after the regulation was incorporated into the EEA Agreement, and hence is also available for any person, company or organization based in Iceland, Liechtenstein and Norway. The TLD is administered by EURid, a consortium originally consisting of the national ccTLD registry operators of Belgium, Sweden, and Italy, joined later by the national registry operator of the Czech Republic. Trademark owners were able to submit registrations through a sunrise period, in an effort to prevent cybersquatting. Full registration started on 7 April 2006.

Health informatics is the study and implementation of computer structures and algorithms to improve communication, understanding, and management of medical information. It can be viewed as branch of engineering and applied science.
A medical classification is used to transform descriptions of medical diagnoses or procedures into standardized statistical code in a process known as clinical coding. Diagnosis classifications list diagnosis codes, which are used to track diseases and other health conditions, inclusive of chronic diseases such as diabetes mellitus and heart disease, and infectious diseases such as norovirus, the flu, and athlete's foot. Procedure classifications list procedure code, which are used to capture interventional data. These diagnosis and procedure codes are used by health care providers, government health programs, private health insurance companies, workers' compensation carriers, software developers, and others for a variety of applications in medicine, public health and medical informatics, including:
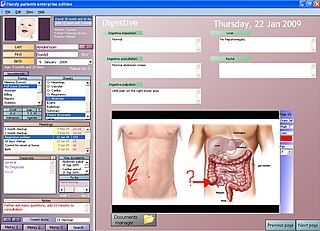
An electronic health record (EHR) is the systematized collection of patient and population electronically stored health information in a digital format. These records can be shared across different health care settings. Records are shared through network-connected, enterprise-wide information systems or other information networks and exchanges. EHRs may include a range of data, including demographics, medical history, medication and allergies, immunization status, laboratory test results, radiology images, vital signs, personal statistics like age and weight, and billing information.
Pharmacovigilance, also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: pharmakon and vigilare. As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended, including lack of efficacy. Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse event, because they may result in an adverse drug reaction.
The ISO/IEC 11179 metadata registry (MDR) standard is an international ISO/IEC standard for representing metadata for an organization in a metadata registry. It documents the standardization and registration of metadata to make data understandable and shareable.
Public health surveillance is, according to the World Health Organization (WHO), "the continuous, systematic collection, analysis and interpretation of health-related data needed for the planning, implementation, and evaluation of public health practice." Public health surveillance may be used to track emerging health-related issues at an early stage and find active solutions in a timely manner. Surveillance systems are generally called upon to provide information regarding when and where health problems are occurring and who is affected.

.me is the Internet country code top-level domain (ccTLD) for Montenegro.
EudraCT is the European clinical trials database of all clinical trials of investigational medicinal products with at least one site in the European Union commencing 1 May 2004 or later. The EudraCT database has been established in accordance with Directive 2001/20/EC. The EudraCT Number is unique and is needed on other documents relating to the trials. No new EudraCT numbers are issued since February 2023. They have been replaced by EU CT numbers.
Ocrelizumab, sold under the brand name Ocrevus, is a medication used for the treatment of multiple sclerosis (MS). It is a humanized anti-CD20 monoclonal antibody. It targets CD20 marker on B lymphocytes and is an immunosuppressive drug. Ocrelizumab binds to an epitope that overlaps with the epitope to which rituximab binds.
Orphanet is an organisation and knowledge base dedicated to rare diseases as well as corresponding diagnosis, orphan drugs, clinical trials and expert networks.
ClinicalTrials.gov is a registry of clinical trials. It is run by the United States National Library of Medicine (NLM) at the National Institutes of Health, and holds registrations from over 444,000 trials from 221 countries.
Healthcare CRM, also known as Healthcare Relationship Management, is a broadly used term for a Customer relationship management system, or CRM, used in healthcare.
Preregistration is the practice of registering the hypotheses, methods, and/or analyses of a scientific study before it is conducted. Clinical trial registration is similar, although it may not require the registration of a study's analysis protocol. Finally, registered reports include the peer review and in principle acceptance of a study protocol prior to data collection.

The Solidarity trial for treatments is a multinational Phase III-IV clinical trial organized by the World Health Organization (WHO) and partners to compare four untested treatments for hospitalized people with severe COVID-19 illness. The trial was announced 18 March 2020, and as of 6 August 2021, 12,000 patients in 30 countries had been recruited to participate in the trial.

Clinical Trials Registry – India (CTRI) is the government of India's official clinical trial registry. The National Institute of Medical Statistics of the Indian Council of Medical Research established the CTRI on 20 July 2007. Since 2009 the Central Drugs Standard Control Organisation has mandated that anyone conducting clinical trials in India must preregister before enrolling any research participants.
References
- ↑ "WHO | About the WHO ICTRP". WHO. Retrieved 2020-10-17.
- ↑ Karam, Ghassan (2020-05-15). "The WHO International Clinical Trials Registry Platform: Providing global clinical trial information to all". On Medicine. Retrieved 2020-10-17.
- ↑ "WHO: International Clinical Trials Registry Platform". GEN - Genetic Engineering and Biotechnology News. 2020-09-01. Retrieved 2020-10-17.
- ↑ "WHO | WHO Data Set". WHO. Archived from the original on November 16, 2011. Retrieved 2020-10-17.
- ↑ "WHO | The Universal Trial Number (UTN)". WHO. Archived from the original on March 26, 2012. Retrieved 2020-10-17.
- ↑ "Clinical trials in India: ethical concerns". Bulletin of the World Health Organization. 86 (8): 581–2. August 2008. doi: 10.2471/blt.08.010808 . PMC 2649459 . PMID 18797610.
- ↑ "Primary registries". www.who.int. Retrieved 2024-04-18.
- ↑ "Data providers". www.who.int. Retrieved 2024-04-18.