
Penicillins are a group of β-lactam antibiotics originally obtained from Penicillium moulds, principally P. chrysogenum and P. rubens. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G and penicillin V. Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use.
Vitamin E is a group of eight fat soluble compounds that include four tocopherols and four tocotrienols. Vitamin E deficiency, which is rare and usually due to an underlying problem with digesting dietary fat rather than from a diet low in vitamin E, can cause nerve problems. Vitamin E is a fat-soluble antioxidant which may help protect cell membranes from reactive oxygen species. Worldwide, government organizations recommend adults consume in the range of 3 to 15 mg per day. As of 2016, consumption was below recommendations according to a worldwide summary of more than one hundred studies that reported a median dietary intake of 6.2 mg per day for alpha-tocopherol.
Tocopherols are a class of organic compounds comprising various methylated phenols, many of which have vitamin E activity. Because the vitamin activity was first identified in 1936 from a dietary fertility factor in rats, it was named tocopherol, from Greek τόκοςtókos 'birth' and φέρεινphérein 'to bear or carry', that is 'to carry a pregnancy', with the ending -ol signifying its status as a chemical alcohol.

Cod liver oil is a dietary supplement derived from liver of cod fish (Gadidae). As with most fish oils, it contains the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and also vitamin A and vitamin D. Historically, it was given to children because vitamin D had been shown to prevent rickets, a consequence of vitamin D deficiency.

Prolactin (PRL), also known as lactotropin and mammotropin, is a protein best known for its role in enabling mammals to produce milk. It is influential in over 300 separate processes in various vertebrates, including humans. Prolactin is secreted from the pituitary gland in response to eating, mating, estrogen treatment, ovulation and nursing. It is secreted heavily in pulses in between these events. Prolactin plays an essential role in metabolism, regulation of the immune system and pancreatic development.
Reference ranges for blood tests are sets of values used by a health professional to interpret a set of medical test results from blood samples. Reference ranges for blood tests are studied within the field of clinical chemistry, the area of pathology that is generally concerned with analysis of bodily fluids.

Cholecalciferol, also known as vitamin D3 and colecalciferol, is a type of vitamin D that is made by the skin when exposed to sunlight; it is found in some foods and can be taken as a dietary supplement.

Ergocalciferol, also known as vitamin D2 and nonspecifically calciferol, is a type of vitamin D found in food and used as a dietary supplement. As a supplement it is used to prevent and treat vitamin D deficiency. This includes vitamin D deficiency due to poor absorption by the intestines or liver disease. It may also be used for low blood calcium due to hypoparathyroidism. It is used by mouth or injection into a muscle.
The enzyme unit, or international unit for enzyme is a unit of enzyme's catalytic activity.

β-Carotene (beta-carotene) is an organic, strongly colored red-orange pigment abundant in fungi, plants, and fruits. It is a member of the carotenes, which are terpenoids (isoprenoids), synthesized biochemically from eight isoprene units and thus having 40 carbons.

The prothrombin time (PT) – along with its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) – is an assay for evaluating the extrinsic pathway and common pathway of coagulation. This blood test is also called protime INR and PT/INR. They are used to determine the clotting tendency of blood, in such things as the measure of warfarin dosage, liver damage, and vitamin K status. PT measures the following coagulation factors: I (fibrinogen), II (prothrombin), V (proaccelerin), VII (proconvertin), and X.
Oxygen radical absorbance capacity (ORAC) was a method of measuring antioxidant capacities in biological samples in vitro. Because no physiological proof in vivo existed in support of the free-radical theory or that ORAC provided information relevant to biological antioxidant potential, it was withdrawn in 2012.

Vitamin D toxicity, or hypervitaminosis D is the toxic state of an excess of vitamin D. The normal range for blood concentration in adults is 20 to 50 nanograms per milliliter (ng/mL).

α-Tocopherol (alpha-tocopherol) is a type of vitamin E. Its E number is "E307". Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals and a hydrophobic side chain which allows for penetration into biological membranes. Compared to the others, α-tocopherol is preferentially absorbed and accumulated in humans.
The Kjeldahl method or Kjeldahl digestion (Danish pronunciation:[ˈkʰelˌtɛˀl]) in analytical chemistry is a method for the quantitative determination of a sample's organic nitrogen plus ammonia/ammonium. (NH3/NH4+). Without modification, other forms of inorganic nitrogen, for instance nitrate, are not included in this measurement. Using an empirical relation between Kjeldahl nitrogen and protein, it is an important method for indirectly quantifying protein content of a sample. This method was developed by Johan Kjeldahl in 1883.

Certified reference materials (CRMs) are 'controls' or standards used to check the quality and metrological traceability of products, to validate analytical measurement methods, or for the calibration of instruments. A certified reference material is a particular form of measurement standard.
Vitamins occur in a variety of related forms known as vitamers. A vitamer of a particular vitamin is one of several related compounds that performs the functions of said vitamin and prevents the symptoms of deficiency of said vitamin.
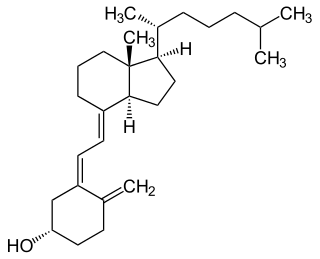
Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and for many other biological effects. In humans, the most important compounds in this group are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol).
Nepidermin, also known as recombinant human epidermal growth factor (rhEGF), is a recombinant form of human epidermal growth factor (EGF) and a cicatrizant. As a recombinant form of EGF, nepidermin is an agonist of the epidermal growth factor receptor (EGFR), and is the first EGFR agonist to be marketed. It was developed by Cuban Center for Genetic Engineering and Biotechnology (CIBG), and has been marketed by Heber Biotech as an intralesional injection for diabetic foot ulcer under the trade name Heberprot-P since 2006. As of 2016, Heberprot-P had been marketed in 23 countries, but remains unavailable in the United States. In 2015, preparations were made to conduct the Phase III trials required for FDA approval, however as of 2023 developments in U.S.-Cuba relations have stymied importation of the drug from Cuba.
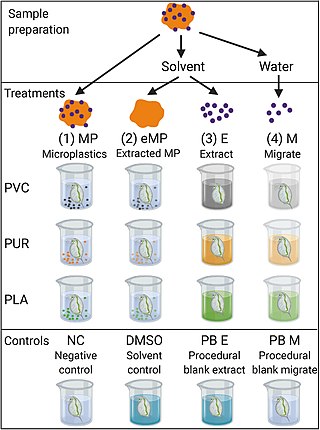
A bioassay is an analytical method to determine the potency or effect of a substance by its effect on living animals or plants, or on living cells or tissues. A bioassay can be either quantal or quantitative, direct or indirect. If the measured response is binary, the assay is quantal; if not, it is quantitative.












