
Metal–organic frameworks (MOFs) are a class of compounds consisting of metal ions or clusters coordinated to organic ligands to form one-, two-, or three-dimensional structures. They are a subclass of coordination polymers, with the special feature that they are often porous. The organic ligands included are sometimes referred to as "struts" or "linkers", one example being 1,4-benzenedicarboxylic acid (BDC).

Zeolitic imidazolate frameworks (ZIFs) are a class of metal-organic frameworks (MOFs) that are topologically isomorphic with zeolites. ZIF glasses can be synthesized by the melt-quench method, and the first melt-quenched ZIF glass was firstly made and reported by Bennett et al. back in 2015. ZIFs are composed of tetrahedrally-coordinated transition metal ions connected by imidazolate linkers. Since the metal-imidazole-metal angle is similar to the 145° Si-O-Si angle in zeolites, ZIFs have zeolite-like topologies. As of 2010, 105 ZIF topologies have been reported in the literature. Due to their robust porosity, resistance to thermal changes, and chemical stability, ZIFs are being investigated for applications such as carbon dioxide capture.
Covalent organic frameworks (COFs) are a class of materials that form two- or three- dimensional structures through reactions between organic precursors resulting in strong, covalent bonds to afford porous, stable, and crystalline materials. COFs emerged as a field from the overarching domain of organic materials as researchers optimized both synthetic control and precursor selection. These improvements to coordination chemistry enabled non-porous and amorphous organic materials such as organic polymers to advance into the construction of porous, crystalline materials with rigid structures that granted exceptional material stability in a wide range of solvents and conditions. Through the development of reticular chemistry, precise synthetic control was achieved and resulted in ordered, nano-porous structures with highly preferential structural orientation and properties which could be synergistically enhanced and amplified. With judicious selection of COF secondary building units (SBUs), or precursors, the final structure could be predetermined, and modified with exceptional control enabling fine-tuning of emergent properties. This level of control facilitates the COF material to be designed, synthesized, and utilized in various applications, many times with metrics on scale or surpassing that of the current state-of-the-art approaches.

Solvothermal synthesis is a method of producing chemical compounds. It is very similar to the hydrothermal route. Both are typically conducted in a stainless steel autoclave. The only difference being that the precursor solution is usually non-aqueous. Typical solvents include dimethylformamide and various alcohols.
Mesoporous organosilica are a type of silica containing organic groups that give rise to mesoporosity. They exhibit pore size ranging from 2 nm - 50 nm, depending on the organic substituents. In contrast, zeolites exhibit pore sizes less than a nanometer. PMOs have potential applications as catalysts, adsorbents, trapping agents, drug delivery agents, stationary phases in chromatography and chemical sensors.

Hong-Cai (Joe) Zhou is a Chinese-American chemist and academic. He is the Davidson Professor of Science and Robert A. Welch Chair in Chemistry at Texas A&M University. He is the associate editor of the journal Inorganic Chemistry.
Kim R. Dunbar is an American inorganic chemist and Distinguished Professor of Chemistry at Texas A&M University. Her research concerns inorganic and coordination chemistry, including molecular magnetism, metals in medicine, supramolecular chemistry Involving anions and anion-pi interactions, and multifunctional materials with organic radicals.
Che Chi-ming, is a Hong Kong chemist currently holding Zhou Guangzhao Professorship in Natural Sciences, following a Dr. Hui Wai-Haan's Chair of Chemistry at the University of Hong Kong (HKU). In 1995, he became the first scientist from Hong Kong to be elected as a member of Chinese Academy of Sciences. He is known for extensive work in inorganic chemistry, photochemistry, and medicinal chemistry.

Mircea Dincă is a Romanian-American inorganic chemist. He is a Professor of Chemistry and W. M. Keck Professor of Energy at the Massachusetts Institute of Technology (MIT). At MIT, Dincă leads a research group that focuses on the synthesis of functional metal-organic frameworks (MOFs), which possess conductive, catalytic, and other material-favorable properties.
Light harvesting materials harvest solar energy that can then be converted into chemical energy through photochemical processes. Synthetic light harvesting materials are inspired by photosynthetic biological systems such as light harvesting complexes and pigments that are present in plants and some photosynthetic bacteria. The dynamic and efficient antenna complexes that are present in photosynthetic organisms has inspired the design of synthetic light harvesting materials that mimic light harvesting machinery in biological systems. Examples of synthetic light harvesting materials are dendrimers, porphyrin arrays and assemblies, organic gels, biosynthetic and synthetic peptides, organic-inorganic hybrid materials, and semiconductor materials. Synthetic and biosynthetic light harvesting materials have applications in photovoltaics, photocatalysis, and photopolymerization.
Natalia B. Shustova is a Peter and Bonnie McCausland Professor of Chemistry at the University of South Carolina. She focuses on developing materials for sustainable energy conversion, metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and graphitic supramolecular structures.
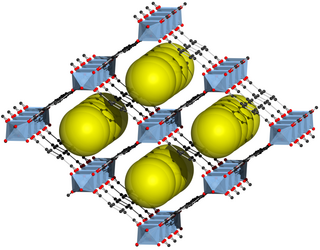
MIL-53 belongs to the class of metal-organic framework (MOF) materials. The first synthesis and the name was established by the group of Gérard Férey in 2002. The MIL-53 structure consists of inorganic [M-OH] chains, which are connected to four neighboring inorganic chains by therephthalate-based linker molecules. Each metal center is octahedrally coordinated by six oxygen atoms. Four of these oxygen atoms originate from four different carboxylate groups and the remaining two oxygen atoms belong to two different μ-OH moieties, which bridge neighboring metal centers. The resulting framework structure contains one-dimensional diamond-shaped pores. Many research group have investigated the flexibility of the MIL-53 structure. This flexible behavior, during which the pore cross-section changes reversibly, was termed 'breathing.effect' and describes the ability of the MIL-53 framework to respond to external stimuli.

Wendy Lee Queen is an American chemist and material scientist. Her research interest focus on development design and production of hybrid organic/inorganic materials at the intersection of chemistry, chemical engineering and material sciences. As of 2020 she is a tenure-track assistant professor at the École polytechnique fédérale de Lausanne (EPFL) in Switzerland, where she directs the Laboratory for Functional Inorganic Materials.

The Hoffmann Institute of Advanced Materials (HIAM) is a science research institute affiliated to Shenzhen Polytechnic in Shenzhen, China. As the eighth institute at Shenzhen named after a Nobel laureate, it was founded in February 2018 under the tutelage of the theoretical chemist Roald Hoffmann. The institute was officially opened with a formal ceremony in May 2019.

Marinella Mazzanti is an Italian inorganic chemist specialized in coordination chemistry. She is a professor at EPFL and the head of the group of Coordination Chemistry at EPFL's School of Basic Sciences.

The oxalate phosphates are chemical compounds containing oxalate and phosphate anions. They are also called oxalatophosphates or phosphate oxalates. Some oxalate-phosphate minerals found in bat guano deposits are known. Oxalate phosphates can form metal organic framework compounds.
A phosphate phosphite is a chemical compound or salt that contains phosphate and phosphite anions (PO33- and PO43-). These are mixed anion compounds or mixed valence compounds. Some have third anions.
The oxalate phosphites are chemical compounds containing oxalate and phosphite anions. They are also called oxalatophosphites or phosphite oxalates. Oxalate phosphates can form metal organic framework compounds.

Fred Wudl is an American materials scientist, academic researcher. He is a Professor Emeritus in the Department of Materials Engineering at the University of California, Santa Barbara.
Amanda Morris is an American chemist who is the Patricia Caldwell Faculty Fellow and Professor of Inorganic and Energy Chemistry at Virginia Tech. Her research considers next-generation materials for catalysis and light-harvesting. She was elected Chair of the American Chemical Society Gay and Transgender Chemists and Allies committee in 2021.










