Related Research Articles

Enthalpy is a property of a thermodynamic system, and is defined as the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant pressure, that is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work required to establish the system's physical dimensions, i.e. to make room for it by displacing its surroundings. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it.

Ferromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism is the strongest type and is responsible for the common phenomenon of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism—paramagnetism, diamagnetism, and antiferromagnetism—but the forces are usually so weak that they can be detected only by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is "the quality of magnetism first apparent to the ancient world, and to us today".

Magnetism is a class of physical attributes that are mediated by magnetic fields. Electric currents and the magnetic moments of elementary particles give rise to a magnetic field, which acts on other currents and magnetic moments. Magnetism is one aspect of the combined phenomenon of electromagnetism. The most familiar effects occur in ferromagnetic materials, which are strongly attracted by magnetic fields and can be magnetized to become permanent magnets, producing magnetic fields themselves. Demagnetizing a magnet is also possible. Only a few substances are ferromagnetic; the most common ones are iron, cobalt and nickel and their alloys. The rare-earth metals neodymium and samarium are less common examples. The prefix ferro- refers to iron, because permanent magnetism was first observed in lodestone, a form of natural iron ore called magnetite, Fe3O4.

A timeline of events in the history of thermodynamics.

James Prescott Joule was an English physicist, mathematician and brewer, born in Salford, Lancashire. Joule studied the nature of heat, and discovered its relationship to mechanical work. This led to the law of conservation of energy, which in turn led to the development of the first law of thermodynamics. The SI derived unit of energy, the joule, is named after him.
In thermodynamics, the Joule–Thomson effect describes the temperature change of a real gas or liquid when it is forced through a valve or porous plug while keeping it insulated so that no heat is exchanged with the environment. This procedure is called a throttling process or Joule–Thomson process. At room temperature, all gases except hydrogen, helium, and neon cool upon expansion by the Joule–Thomson process when being throttled through an orifice; these three gases experience the same effect but only at lower temperatures. Most liquids such as hydraulic oils will be warmed by the Joule–Thomson throttling process.
Sensible heat is heat exchanged by a body or thermodynamic system in which the exchange of heat changes the temperature of the body or system, and some macroscopic variables of the body or system, but leaves unchanged certain other macroscopic variables of the body or system, such as volume or pressure.

Latent heat is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process — usually a first-order phase transition.
Magnetostriction is a property of magnetic materials that causes them to change their shape or dimensions during the process of magnetization. The variation of materials' magnetization due to the applied magnetic field changes the magnetostrictive strain until reaching its saturation value, λ. The effect was first identified in 1842 by James Joule when observing a sample of iron.
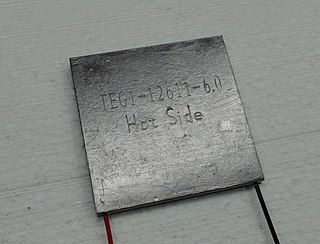
The thermoelectric effect is the direct conversion of temperature differences to electric voltage and vice versa via a thermocouple. A thermoelectric device creates a voltage when there is a different temperature on each side. Conversely, when a voltage is applied to it, heat is transferred from one side to the other, creating a temperature difference. At the atomic scale, an applied temperature gradient causes charge carriers in the material to diffuse from the hot side to the cold side.

The term 'thermal energy' is used differently, and often loosely, in different contexts. It refers to several distinct physical concepts, such as the internal energy, or as the enthalpy, of a body of matter and radiation; or as heat, defined as a type of energy transfer ; or as the characteristic energy of a degree of freedom, , in a system that is described in terms of its microscopic particulate constituents, where denotes temperature and denotes the Boltzmann constant.

In science, a process that is not reversible is called irreversible. This concept arises frequently in thermodynamics.

Jean Charles Athanase Peltier was a French physicist. He was originally a watch dealer, but at the age of 30 began experiments and observations in physics.
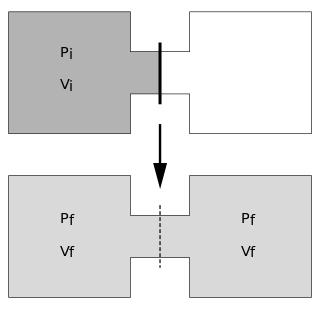
The Joule expansion is an irreversible process in thermodynamics in which a volume of gas is kept in one side of a thermally isolated container, with the other side of the container being evacuated. The partition between the two parts of the container is then opened, and the gas fills the whole container.

The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general. Owing to the relevance of thermodynamics in much of science and technology, its history is finely woven with the developments of classical mechanics, quantum mechanics, magnetism, and chemical kinetics, to more distant applied fields such as meteorology, information theory, and biology (physiology), and to technological developments such as the steam engine, internal combustion engine, cryogenics and electricity generation. The development of thermodynamics both drove and was driven by atomic theory. It also, albeit in a subtle manner, motivated new directions in probability and statistics; see, for example, the timeline of thermodynamics.

In thermodynamics, work performed by a system is energy transferred by the system to its surroundings, by a mechanism through which the system can spontaneously exert macroscopic forces on its surroundings. In the surroundings, through suitable passive linkages, the work can lift a weight, for example. Energy can also transfer from the surroundings to the system; in a sign convention used in physics, such work has a negative magnitude.
The Gough–Joule effect is originally the tendency of elastomers to contract when heated if they are under tension. Elastomers that are not under tension do not see this effect. The term is also used more generally to refer to the dependence of the temperature of any solid on the mechanical deformation. This effect can be observed in nylon strings of classical guitars, whereby the string contracts as a result of heating.
John Gough was a blind English natural and experimental philosopher who is known for his own investigations as well as the influence he had on both John Dalton and William Whewell.

In thermodynamics, heat is energy in transfer to or from a thermodynamic system, by mechanisms other than thermodynamic work or transfer of matter. The various mechanisms of energy transfer that define heat are stated in the next section of this article.
The joule is the SI derived unit of energy
References
- ↑ Crew, Henry (1910). General physics: an elementary text-book FOR colleges, 2nd Edition. The University of Michigan: The Macmillan Company. pp. 402–404.
- ↑ Joule, J.P. (1847). "On the Effects of Magnetism upon the Dimensions of Iron and Steel Bars". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. Taylor & Francis. 30, Third Series: 76–87, 225–241. Retrieved 2009-07-19. Joule observed in this paper that he first reported the measurements in a "Conversazione" in Manchester, England, in (Sturgeon's) Annals of Electricity, Magnetism, and Chemistry 8, 219-224 (1842)
- ↑ Longair, M. S. (2003). Theoretical concepts in physics: an alternative view of theoretical reasoning in physics, 2nd Ed. Cambridge University Press. p. 217. ISBN 978-0-521-52878-8.
- ↑ "Thermodynamics of cooling" (PDF). The University of Sydney. 2005. Archived from the original (PDF) on 2011-06-11. Retrieved 2009-07-22.
- ↑ "John Gough and his Observation of Rubber Thermodynamics". Yale University. 1998-10-06. Archived from the original on 2011-06-07. Retrieved 2009-07-19.
- ↑ Loadman, John (2005). Tears of the Tree: The Story of Rubber -- A Modern Marvel. Oxford University Press. p. 165. ISBN 978-0-19-856840-7.
- ↑ Kouhoupt, Rudy (January 1972). Heat Runs. Popular Science. Retrieved 2009-07-20.
- ↑ Nagdi, Khairi (1992). Rubber as an Engineering Material. Hanser Verlag. pp. 33–34. ISBN 978-3-446-16282-2.