Research
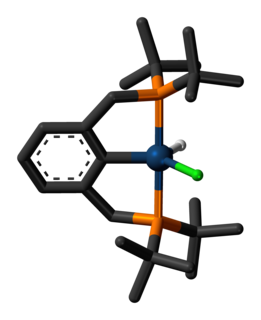
Goldberg's research interests include understanding the mechanism and application of catalysts on fundamental organometallic reactions. This culminates into a goal to design more efficient, cheaper, and greener chemical products and fuels from a variety of feedstocks, such as alkanes. One such process that Goldberg has helped develop is the dehydrogenation of ammonia borane using an iridium pincer catalyst, a reaction that took place under mild conditions at high rates with efficient catalyst regeneration. [5]
Electrophilic Oxidation Catalysis
Over thirty years ago, Shilov discovered the selective oxidization of alkanes in the presence of platinum-based metals. This was impractical because it required a stoichiometric oxidant in addition to the catalytic Pt(II) metal, which led Goldberg to inquire deeper into understanding C-H bond activation, oxidation, and C-heteroatom bond formation, leading to the development of more practical products. In recent studies of utilizing alkanes, Goldberg has investigated the functionalization of alkanes through oxidation reactions using platinum-based catalysts. [6]

Pt(II) methyl complexes are key intermediates in both the Shilov methane oxidation system and in more recent catalytic Pt methane oxidation systems. Goldberg's research involves formation of alcohols from alkanes using platinum or other late metal catalysts, including ruthenium, iridium and rhodium. As a result, her research discovered a method of using a family of Ru(II) diamine complexes as a precatalyst to provide selectivity and high conversion of aldehydes to carboxylic acids over the competing aldehyde disproportionation reaction. [7]
Lithium aluminum hydride has widely been used as strong reducing reagent. However, it is difficult to reduce resonance stabilized carbonyl groups present in esters and lactones to alcohols. It was then that her research group came up with idea of hydrogenation of esters and lactones to form alcohol using base-free metal-catalyzed complexes. The catalyst that gave rise to a high yield of formate esters is a half-sandwich iridium bipyridine complex. The same half-sandwich complexes of iridium and rhodium were used as competent catalysts to hydrogenate carboxylic acids under relatively mild conditions. The mechanism behind this reaction involves hydride transfer from catalyst to formic acid as the main part of the reaction. [8]
Through the Center for Enabling New Technology through Catalysis (CENTC), [9] Goldberg also contributed to finding methods of activating strong bonds such as C-H, C-C, C-O, C-N, and N-H. Through this, the Goldberg research group discovered how to functionalize these bonds once they are activated through oxidative addition and reductive elimination. This investigation provided detailed mechanisms, intermediates, and kinetic barriers for these catalytic processes. [6]
Anti-Markovnikov Hydroamination of Alkenes
Recognizing the importance of linear anti-Markovnikov products, Goldberg's research focuses on discovery of transition metal catalysts which helps in catalysis of anti-Markovnikov hydroamination of alkenes. In one of her publications, she introduces a method to catalyze the hydroarylation of unactivated alkenes using Pt(II) complexes with unsymmetrical pyrrolide ligands. Selectivity was provided by using benzene and 1-hexene and an optimized catalyst. The result was production of high olefin concentration using propylene as the substrate. [10]
Most of her research on the subject has involved experimental studies of reductive elimination and oxidative addition reactions involving carbon-containing molecules in order to gain information on the reaction coordinates of such processes. Her further studies on using platinum-based catalysts for the reductive eliminations of alkane products have also included crystallography characterizations of platinum complexes and selected intermediates to determine the mechanism of such reactions. [11]
Molecular Oxygen Catalysis

Goldberg's research interests also include harnessing molecular oxygen as a selective oxidant in catalysis. As molecular oxygen is readily available and environmentally benign, the Goldberg group, along with other research groups involved in the CENTC, have attempted to better comprehend oxygen's reactivity and to activate it to utilize it to its fullest capacity. Current research has aimed to understand how reactions between transition metal complexes and oxygen occur. Goldberg has recently investigated the insertion of molecular oxygen into palladium-hydride bonds, with results suggesting that this insertion reaction does not involve radical chain mechanisms. [12] This research of the capabilities of oxygen to insert into palladium-hydride bonds have been expanded by the study of the general reactivity of molecular oxygen with middle- to late-transition metals, such as platinum. [13] This contribution to the understanding of molecular oxygen's method of reaction with palladium and other transition metals may lead to further development and perfecting of molecular oxygen as a selective oxidizer.
Gem-Dialkyl Ligands

Further research by Goldberg on the study of transition metal-catalyzed reactions places additional emphasis on the metal-complex ligands. Recent publications have reported that gem-dialkyl substituents on platinum-based metal complexes can be utilized to determine the mechanism of reaction pathway and whether the mechanism includes chelate opening. [14] The gem-dialkyl substituents have been used in the past for recognizing thermodynamic properties of chemical systems, though recent studies have pushed those discoveries to the understanding of kinetic systems as well. Goldberg's research on the effects of these types of substituents on bidentate ligands and how these effects change the mechanisms and rates of reductive elimination reactions has helped advance improvements of transition metal-based inorganic and organic catalysis.