Related Research Articles

Redox is a type of chemical reaction in which the oxidation states of a substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state.
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease.

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.
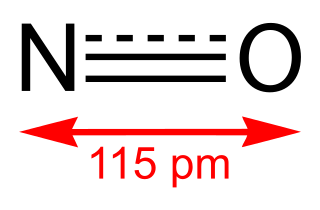
Nitric oxide is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula. Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is now being appreciated, siderophores are among the strongest (highest affinity) Fe3+ binding agents known. Phytosiderophores are siderophores produced by plants.
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well as artificially introduced metals, including those that are non-essential, in medicine and toxicology. Many biological processes such as respiration depend upon molecules that fall within the realm of inorganic chemistry. The discipline also includes the study of inorganic models or mimics that imitate the behaviour of metalloproteins.

In chemistry, an ionophore is a chemical species that reversibly binds ions. Many ionophores are lipid-soluble entities that transport ions across the cell membrane. Ionophores catalyze ion transport across hydrophobic membranes, such as liquid polymeric membranes or lipid bilayers found in the living cells or synthetic vesicles (liposomes). Structurally, an ionophore contains a hydrophilic center and a hydrophobic portion that interacts with the membrane.

Heme oxygenase, or haem oxygenase, is an enzyme that catalyzes the degradation of heme to produce biliverdin, ferrous ion, and carbon monoxide.
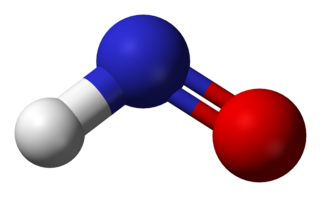
Nitroxyl or azanone is the chemical compound HNO. It is well known in the gas phase. Nitroxyl can be formed as a short-lived intermediate in the solution phase. The conjugate base, NO−, nitroxide anion, is the reduced form of nitric oxide (NO) and is isoelectronic with dioxygen. The bond dissociation energy of H−NO is 49.5 kcal/mol (207 kJ/mol), which is unusually weak for a bond to the hydrogen atom.
A nitrate test is a chemical test used to determine the presence of nitrate ion in solution. Testing for the presence of nitrate via wet chemistry is generally difficult compared with testing for other anions, as almost all nitrates are soluble in water. In contrast, many common ions give insoluble salts, e.g. halides precipitate with silver, and sulfate precipitate with barium.

Sodium diethyldithiocarbamate is the organosulfur compound with the formula NaS2CN(C2H5)2. It is a pale yellow, water soluble salt.
Pro-oxidants are chemicals that induce oxidative stress, either by generating reactive oxygen species or by inhibiting antioxidant systems. The oxidative stress produced by these chemicals can damage cells and tissues, for example, an overdose of the analgesic paracetamol (acetaminophen) can fatally damage the liver, partly through its production of reactive oxygen species.

Pyrrolidine dithiocarbamate (PDTC) are a family of closely related drugs used for a metal chelation, induction of G1 phase cell cycle arrest, and preventing induction of nitric oxide synthase.

Stephen James Lippard is the Arthur Amos Noyes Emeritus Professor of Chemistry at the Massachusetts Institute of Technology. He is considered one of the founders of bioinorganic chemistry, studying the interactions of nonliving substances such as metals with biological systems. He is also considered a founder of metalloneurochemistry, the study of metal ions and their effects in the brain and nervous system. He has done pioneering work in understanding protein structure and synthesis, the enzymatic functions of methane monooxygenase (MMO), and the mechanisms of cisplatin anticancer drugs. His work has applications for the treatment of cancer, for bioremediation of the environment, and for the development of synthetic methanol-based fuels.
Methanobactin (mb) is a class of copper-binding and reducing chromophoric peptides initially identified in the methanotroph Methylococcus capsulatus Bath - and later in Methylosinus trichosporium OB3b - during the isolation of the membrane-associated or particulate methane monooxygenase (pMMO). It is thought to be secreted to the extracellular media to recruit copper, a critical component of methane monooxygenase, the first enzyme in the series that catalyzes the oxidation of methane into methanol. Methanobactin functions as a chalkophore, similar to iron siderophores, by binding to Cu(II) or Cu(I) then shuttling the copper into the cell. Methanobactin has an extremely high affinity for binding and Cu(I) with a Kd of approximately 1020 M−1 at pH 8. Additionally, methanobactin can reduce Cu(II), which is toxic to cells, to Cu(I), the form used in pMMO. Moreover, different species of methanobactin are hypothesized to be ubiquitous within the biosphere, especially in light of the discovery of molecules produced by other type II methanotrophs that similarly bind and reduce copper (II) to copper (I).
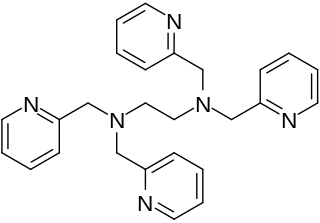
TPEN (N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine) is an intracellular membrane-permeable ion chelator. TPEN has a high affinity for many transition metals and should not be considered specific or selective for a particular ion. Chelators can be used in chelation therapy to remove toxic metals in the body. TPEN is a chelator that has a high affinity for zinc. For example, one study showed that TPEN is a stronger chelator compared to other chelators like pentetic acid (DTPA) when high levels of zinc are present (15 μM). When low levels of zinc were present however (0, 3, 6, 9 and 12 μM zinc), there was no significant difference. TPEN is a hexadentate ligand which also forms complexes with other soft metal ions such as Cd2+.
Alison Butler is a Distinguished Professor in the Department of Chemistry and Biochemistry at the University of California, Santa Barbara. She works on bioinorganic chemistry and metallobiochemistry. She is a Fellow of the American Association for the Advancement of Science (1997), the American Chemical Society (2012), the American Academy of Arts and Sciences (2019), and the Royal Society of Chemistry (2019). She was elected a member of the National Academy of Sciences in 2022.
Katrina Miranda is an associate professor of biochemistry at the University of Arizona. She works on nitric oxide and their role in diseases like breast cancer, stroke and chronic pain.

Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.
Kay Michille Brummond is an American synthetic chemist who is Professor of Chemistry and Associate Dean of Faculty at the University of Pittsburgh. Her interests consider cycloaddition reactions that can realise molecules and natural products for organic photovoltaics and targeted covalent inhibitors. She was elected a Fellow of the American Chemical Society (ACS) in 2010, a Fellow of the AAAS in 2021, and awarded the ACS National Award for Encouraging Women into Careers in the Chemical Sciences in 2021.
References
- 1 2 3 4 5 "People – The Franz Lab" . Retrieved 2019-08-27.
- ↑ "NEWMAC". NEWMAC. Retrieved 2019-08-27.
- ↑ Franz, Katherine J. (Katherine Jennings) (2000). Nitric oxide reactivity of manganese, iron and cobalt tropocoronands and development of fluorescent aminotroponiminates for nitric oxide sensing (Thesis thesis). Massachusetts Institute of Technology. hdl: 1721.1/29590 .
- ↑ "Franz, Katherine J." Duke Cancer Institute. 2016-12-20. Retrieved 2019-08-27.
- ↑ "Dr. Katherine J. Franz - Department of Chemistry & Biochemistry - UMBC". chemistry.umbc.edu. Retrieved 2019-08-27.
- 1 2 3 4 5 "Research – The Franz Lab" . Retrieved 2019-08-27.
- ↑ Bakthavatsalam S, Sleeper ML, Dharani A, George DJ, Zhang T, Franz KJ (September 2018). "Leveraging γ-Glutamyl Transferase To Direct Cytotoxicity of Copper Dithiocarbamates against Prostate Cancer Cells". Angewandte Chemie. 57 (39): 12780–12784. doi:10.1002/anie.201807582. PMC 6372289 . PMID 30025197.
- ↑ Conklin SE, Bridgman EC, Su Q, Riggs-Gelasco P, Haas KL, Franz KJ (August 2017). "Specific Histidine Residues Confer Histatin Peptides with Copper-Dependent Activity against Candida albicans". Biochemistry. 56 (32): 4244–4255. doi:10.1021/acs.biochem.7b00348. PMC 5937946 . PMID 28763199.
- ↑ Having Multiple Mentors: Katherine J. Franz , retrieved 2019-08-27
- ↑ "Katherine J. Franz, 2016 Dean's Award Winner | Duke Graduate School". gradschool.duke.edu. Retrieved 2019-08-27.
- ↑ "ACS 2020 national award winners". Chemical & Engineering News. Retrieved 2019-08-27.
- ↑ "Chemical Reviews | Vol 119, No 2". pubs.acs.org. Retrieved 2019-08-27.