Creutzfeldt–Jakob disease (CJD), also known as subacute spongiform encephalopathy or neurocognitive disorder due to prion disease, is a fatal degenerative brain disorder. Early symptoms include memory problems, behavioral changes, poor coordination, and visual disturbances. Later symptoms include dementia, involuntary movements, blindness, weakness, and coma. About 70% of people die within a year of diagnosis. The name Creutzfeldt–Jakob disease was introduced by Walther Spielmeyer in 1922, after the German neurologists Hans Gerhard Creutzfeldt and Alfons Maria Jakob.

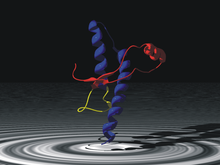
A prion is a misfolded protein that can induce misfolding of normal variants of the same protein and trigger cellular death. Prions cause prion diseases known as transmissible spongiform encephalopathies (TSEs) that are transmissible, fatal neurodegenerative diseases in humans and animals. The proteins may misfold sporadically, due to genetic mutations, or by exposure to an already misfolded protein. The consequent abnormal three-dimensional structure confers on them the ability to cause misfolding of other proteins.

Stanley Ben Prusiner is an American neurologist and biochemist. He is the director of the Institute for Neurodegenerative Diseases at University of California, San Francisco (UCSF). Prusiner discovered prions, a class of infectious self-reproducing pathogens primarily or solely composed of protein, a scientific theory considered by many as a heretical idea when first proposed. He received the Albert Lasker Award for Basic Medical Research in 1994 and the Nobel Prize in Physiology or Medicine in 1997 for research on prion diseases developed by him and his team of experts beginning in the early 1970s.

Scrapie is a fatal, degenerative disease affecting the nervous systems of sheep and goats. It is one of several transmissible spongiform encephalopathies (TSEs), and as such it is thought to be caused by a prion. Scrapie has been known since at least 1732 and does not appear to be transmissible to humans. However, it has been found to be experimentally transmissible to humanised transgenic mice and non-human primates.

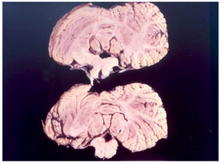
Transmissible spongiform encephalopathies (TSEs) also known as prion diseases, are a group of progressive, incurable, and fatal conditions that are associated with prions and affect the brain and nervous system of many animals, including humans, cattle, and sheep. According to the most widespread hypothesis, they are transmitted by prions, though some other data suggest an involvement of a Spiroplasma infection. Mental and physical abilities deteriorate and many tiny holes appear in the cortex causing it to appear like a sponge when brain tissue obtained at autopsy is examined under a microscope. The disorders cause impairment of brain function, including memory changes, personality changes and problems with movement that worsen chronically.

Chronic wasting disease (CWD), sometimes called zombie deer disease, is a transmissible spongiform encephalopathy (TSE) affecting deer. TSEs are a family of diseases thought to be caused by misfolded proteins called prions and include similar diseases such as BSE in cattle, Creutzfeldt–Jakob disease (CJD) in humans and scrapie in sheep. Natural infection causing CWD affects members of the deer family. In the United States, CWD affects mule deer, white-tailed deer, red deer, sika deer, elk, caribou, and moose. The transmission of CWD to other species such as squirrel monkeys and humanized mice has been observed in experimental settings.

Daniel Carleton Gajdusek was an American physician and medical researcher who was the co-recipient of the Nobel Prize in Physiology or Medicine in 1976 for work on the transmissibility of kuru, implying the existence of an infectious agent, which he named an 'unconventional virus'.
The Fore people live in the Okapa District of the Eastern Highlands Province, Papua New Guinea. There are approximately 20,000 Fore who are separated by the Wanevinti Mountains into the North Fore and South Fore regions. Their main form of subsistence is slash-and-burn farming. The Fore language has three distinct dialects and is the southernmost member of the East Central Family, East New Guinea Highlands Stock, Trans–New Guinea phylum of Papuan languages.

Gerstmann–Sträussler–Scheinker syndrome (GSS) is an extremely rare, always fatal neurodegenerative disease that affects patients from 20 to 60 years in age. It is exclusively heritable, and is found in only a few families all over the world. It is, however, classified with the transmissible spongiform encephalopathies (TSE) due to the causative role played by PRNP, the human prion protein. GSS was first reported by the Austrian physicians Josef Gerstmann, Ernst Sträussler and Ilya Scheinker in 1936.
Transmissible mink encephalopathy (TME) is a rare sporadic disease that affects the central nervous system of ranch-raised adult mink. It is a transmissible spongiform encephalopathy, caused by proteins called prions.

Major prion protein (PrP) is encoded in the human body by the PRNP gene also known as CD230. Expression of the protein is most predominant in the nervous system but occurs in many other tissues throughout the body.
Laura Manuelidis is a physician and neuropathologist at Yale University.

Variant Creutzfeldt–Jakob disease (vCJD), commonly referred to as "mad cow disease" or "human mad cow disease" to distinguish it from its BSE counterpart, is a fatal type of brain disease within the transmissible spongiform encephalopathy family. Initial symptoms include psychiatric problems, behavioral changes, and painful sensations. In the later stages of the illness, patients may exhibit poor coordination, dementia and involuntary movements. The length of time between exposure and the development of symptoms is unclear, but is believed to be years to decades. Average life expectancy following the onset of symptoms is 13 months.
Endocannibalism is a practice of cannibalism in one's own locality or community. In most cases this refers to the consumption of the remains of the deceased in a mortuary context.

Bovine spongiform encephalopathy (BSE), commonly known as mad cow disease, is an incurable and invariably fatal neurodegenerative disease of cattle. Symptoms include abnormal behavior, trouble walking, and weight loss. Later in the course of the disease, the cow becomes unable to function normally. There is conflicting information about the time between infection and onset of symptoms. In 2002, the World Health Organization (WHO) suggested it to be approximately four to five years. Time from onset of symptoms to death is generally weeks to months. Spread to humans is believed to result in variant Creutzfeldt–Jakob disease (vCJD). As of 2018, a total of 231 cases of vCJD had been reported globally.

Variably protease-sensitive prionopathy (VPSPr) is a sporadic prion protein disease first described in an abstract for a conference on prions in 2006, and this study was published in a 2008 report on 11 cases. The study was conducted by Gambetti P., Zou W.Q., and coworkers from the United States National Prion Disease Pathology Surveillance Center. It was first identified as a distinct disease in 2010 by Zou W.Q. and coworkers from the United States National Prion Disease Pathology Surveillance Center.
Frank O. Bastian is an American physician and neuropathologist, who previously worked at Louisiana State University, moved to a university in New Orleans in 2019. He specializes in the transmissible spongiform encephalopathies (TSEs), which include, but are not limited to, Bovine spongiform encephalopathy (BSE) "Mad cow disease" in cattle, scrapie in sheep and goats, and Creutzfeldt–Jakob disease (CJD) in humans.
Shirley Inglis Lindenbaum is an Australian anthropologist notable for her medical anthropology work on kuru in Papua New Guinea, HIV/AIDS in the United States of America, and cholera in Bangladesh.

The United Kingdom was afflicted with an outbreak of Bovine spongiform encephalopathy, and its human equivalent variant Creutzfeldt–Jakob disease (vCJD), in the 1980s and 1990s. Over four million head of cattle were slaughtered in an effort to contain the outbreak, and 178 people died after contracting vCJD through eating infected beef. A political and public health crisis resulted, and British beef was banned from export to numerous countries around the world, with some bans remaining in place until as late as 2019.
Michael Coulthart is a Canadian microbiologist who is employed as the head of the Canadian Creutzfeldt–Jakob Disease Surveillance System (CJDSS) within the Public Health Agency of Canada (PHAC), which terms CJD a zoonotic and infectious disease. In 2006, a working group named "classic CJD" as well as Variant Creutzfeldt–Jakob disease as two notifiable diseases. It is unknown whether PHAC tracks in an official capacity other transmissible spongiform encephalopathies (TSE), but Coulthart is on the Advisory Committee of the Center for Infectious Disease Research and Policy for Chronic Wasting Disease of cervidae.