Related Research Articles
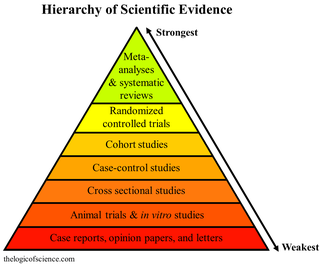
Meta-analysis is the statistical combination of the results of multiple studies addressing a similar research question. An important part of this method involves computing an effect size across all of the studies; this involves extracting effect sizes and variance measures from various studies. Meta-analyses are integral in supporting research grant proposals, shaping treatment guidelines, and influencing health policies. They are also pivotal in summarizing existing research to guide future studies, thereby cementing their role as a fundamental methodology in metascience. Meta-analyses are often, but not always, important components of a systematic review procedure. For instance, a meta-analysis may be conducted on several clinical trials of a medical treatment, in an effort to obtain a better understanding of how well the treatment works.

Cochrane is a British international charitable organisation formed to synthesize medical research findings to facilitate evidence-based choices about health interventions involving health professionals, patients and policy makers. It includes 53 review groups that are based at research institutions worldwide. Cochrane has approximately 30,000 volunteer experts from around the world.

Cardiovascular disease (CVD) is any disease involving the heart or blood vessels. CVDs constitute a class of diseases that includes: coronary artery diseases, heart failure, hypertensive heart disease, rheumatic heart disease, cardiomyopathy, arrhythmia, congenital heart disease, valvular heart disease, carditis, aortic aneurysms, peripheral artery disease, thromboembolic disease, and venous thrombosis.

A systematic review is a scholarly synthesis of the evidence on a clearly presented topic using critical methods to identify, define and assess research on the topic. A systematic review extracts and interprets data from published studies on the topic, then analyzes, describes, critically appraises and summarizes interpretations into a refined evidence-based conclusion. For example, a systematic review of randomized controlled trials is a way of summarizing and implementing evidence-based medicine.
In statistics, (between-) study heterogeneity is a phenomenon that commonly occurs when attempting to undertake a meta-analysis. In a simplistic scenario, studies whose results are to be combined in the meta-analysis would all be undertaken in the same way and to the same experimental protocols. Differences between outcomes would only be due to measurement error. Study heterogeneity denotes the variability in outcomes that goes beyond what would be expected due to measurement error alone.
In epidemiology, reporting bias is defined as "selective revealing or suppression of information" by subjects. In artificial intelligence research, the term reporting bias is used to refer to people's tendency to under-report all the information available.
In natural and social science research, a protocol is most commonly a predefined procedural method in the design and implementation of an experiment. Protocols are written whenever it is desirable to standardize a laboratory method to ensure successful replication of results by others in the same laboratory or by other laboratories. Additionally, and by extension, protocols have the advantage of facilitating the assessment of experimental results through peer review. In addition to detailed procedures, equipment, and instruments, protocols will also contain study objectives, reasoning for experimental design, reasoning for chosen sample sizes, safety precautions, and how results were calculated and reported, including statistical analysis and any rules for predefining and documenting excluded data to avoid bias.
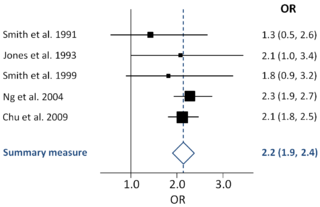
A forest plot, also known as a blobbogram, is a graphical display of estimated results from a number of scientific studies addressing the same question, along with the overall results. It was developed for use in medical research as a means of graphically representing a meta-analysis of the results of randomized controlled trials. In the last twenty years, similar meta-analytical techniques have been applied in observational studies and forest plots are often used in presenting the results of such studies also.

A review article is an article that summarizes the current state of understanding on a topic within a certain discipline. A review article is generally considered a secondary source since it may analyze and discuss the method and conclusions in previously published studies. It resembles a survey article or, in news publishing, overview article, which also surveys and summarizes previously published primary and secondary sources, instead of reporting new facts and results. Survey articles are however considered tertiary sources, since they do not provide additional analysis and synthesis of new conclusions. A review of such sources is often referred to as a tertiary review.
Peter Christian Gøtzsche is a Danish physician, medical researcher, and former leader of the Nordic Cochrane Center at Rigshospitalet in Copenhagen, Denmark. He is a co-founder of the Cochrane Collaboration and has written numerous reviews for the organization. His membership in Cochrane was terminated by its Governing Board of Trustees on 25 September 2018. During the COVID-19 pandemic, Gøtzsche was criticised for spreading disinformation about COVID-19 vaccines.
Critical appraisal in evidence based medicine, is the use of explicit, transparent methods to assess the data in published research, applying the rules of evidence to factors such as internal validity, adherence to reporting standards, conclusions, generalizability and risk-of-bias. Critical appraisal methods form a central part of the systematic review process. They are used in evidence synthesis to assist clinical decision-making, and are increasingly used in evidence-based social care and education provision.

PRISMA is an evidence-based minimum set of items aimed at helping scientific authors to report a wide array of systematic reviews and meta-analyses, primarily used to assess the benefits and harms of a health care intervention. PRISMA focuses on ways in which authors can ensure a transparent and complete reporting of this type of research. The PRISMA standard superseded the earlier QUOROM standard. It offers the replicability of a systematic literature review. Researchers have to figure out research objectives that answer the research question, states the keywords, a set of exclusion and inclusion criteria. In the review stage, relevant articles were searched, irrelevant ones are removed. Articles are analyzed according to some pre-defined categories.
Tom Jefferson is a British epidemiologist, based in Rome, Italy, who works for the Cochrane Collaboration. Jefferson is an author and editor of the Cochrane Collaboration's acute respiratory infections group, as well as part of four other Cochrane groups. He was also an advisor to the Italian National Agency for Regional Health Services.
Isabelle Boutron is a professor of epidemiology at the Université Paris Cité and head of the INSERM- METHODS team within the Centre of Research in Epidemiology and Statistics (CRESS). She was originally trained in rheumatology and later switched to a career in epidemiology and public health. She is also deputy director of the French EQUATOR Centre, member of the SPIRIT-CONSORT executive committee, director of Cochrane France and co-convenor of the Bias Methods group of the Cochrane Collaboration.

Cynthia Mulrow is an American physician and scholar from Edinburg, Texas. She has regularly contributed academic research on many topics to the medical community. Her academic work mainly focuses on systematic reviews and evidence reports, research methodology, and chronic medical conditions.
Kay Dickersin is an academic who trained first in cell biology and subsequently epidemiology. She went on to a career studying factors that influence research integrity, in particular publication bias and outcome reporting bias. She is retired Professor Emerita in the Department of Epidemiology at Johns Hopkins Bloomberg School of Public Health where she was Director of the Center for Clinical Trials and Evidence Synthesis there. She was also Director of the US Cochrane Center and the US Satellite of the Cochrane Eyes and Vision Group within the Cochrane Collaboration. Dickersin received multiple awards for her research.
Individual participant data is raw data from individual participants, and is often used in the context of meta-analysis.
Allegiance bias in behavioral sciences is a bias resulted from the investigator's or researcher's allegiance to a specific school of thought. Researchers/investigators have been exposed to many types of branches of psychology or schools of thought. Naturally they adopt a school or branch that fits with their paradigm of thinking. More specifically, allegiance bias is when this leads therapists, researchers, etc. believing that their school of thought or treatment is superior to others. Their superior belief to these certain schools of thought can bias their research in effective treatments trials or investigative situations leading to allegiance bias. Reason being is that they may have devoted their thinking to certain treatments they have seen work in their past experiences. This can lead to errors in interpreting the results of their research. Their “pledge” to stay within their own paradigm of thinking may affect their ability to find more effective treatments to help the patient or situation they are investigating.
The International Prospective Register of Systematic Reviews, better known as PROSPERO, is an open access online database of systematic review protocols on a wide range of topics. While it was initially restricted to medicine, as of 2021, it also accepts protocols in criminology, social care, education and international development, as long as there is a health-related outcome. Researchers can choose to have their reviews prospectively registered with PROSPERO. The database is produced by the Centre for Reviews and Dissemination at the University of York in England, and it is funded by the National Institute for Health Research. Registration of systematic reviews in the database has been supported by PLoS Medicine, BioMed Central, the EQUATOR Network, and BMJ editor-in-chief Fiona Godlee, among others.
non-pharmacological intervention (NPI) is any type of healthcare intervention which is not primarily based on medication. Some examples include exercise, sleep improvement, and dietary habits.
References
- ↑ "Welcome to the IPD Meta-analysis Methods Group". Cochrane Individual Participant Data (IPD) Meta-analysis Methods Group. The Cochrane Collaboration. 6 January 2015. Retrieved 23 May 2015.
- ↑ Stewart, LA; Parmar, MKB (1993). "Meta-analysis of the literature or of individual patient data: is there a difference?". Lancet. 341 (8842): 418–422. doi:10.1016/0140-6736(93)93004-K. PMID 8094183. S2CID 34704861.
- ↑ "Centre for Reviews and Dissemination". The University of York Centre for Reviews and Dissemination. University of York. 2015. Retrieved 23 May 2015.
- 1 2 Stewart, LA; Clarke, M; Rovers, M; Riley, RD; Simmonds, M; Stewart, G; Tierney, JF; PRISMA-IPD, Development Group (2015). "Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement". JAMA. 313 (16): 1657–1665. doi: 10.1001/jama.2015.3656 . PMID 25919529.
- ↑ Stewart, GB; Altman, DG; Askie, LM; Duley, L; Simmonds, MC; Stewart, LA (2012). "Statistical analysis of individual participant data meta-analyses: a comparison of methods and recommendations for practice". PLOS ONE. 7 (10): e46042. Bibcode:2012PLoSO...746042S. doi: 10.1371/journal.pone.0046042 . PMC 3463584 . PMID 23056232.
- ↑ Simmonds, MC; Higgins, JP; Stewart, LA; Tierney, JF; Clarke, MJ; Thompson, SG (2005). "Meta-analysis of individual patient data from randomized trials: a review of methods used in practice". Clin Trials. 2 (3): 209–217. doi:10.1191/1740774505cn087oa. PMID 16279144. S2CID 24916211.
- ↑ Tierney, JF; Stewart, LA (2005). "Investigating patient exclusion bias in meta-analysis". Int J Epidemiol. 34 (1): 79–87. doi: 10.1093/ije/dyh300 . PMID 15561753.
- ↑ Stewart, LA; Tierney, JF (2002). "To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data". Eval Health Prof. 25 (1): 76–97. doi:10.1177/0163278702025001006. PMID 11868447. S2CID 1323552.
- ↑ Moher, D; Shamseer, L; Clarke, M; Ghersi, D; Liberati, A; Petticrew, M; Shekelle, P; Stewart, LA; PRISMA-P, Group (2015). "Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement". Syst Rev. 4 (1): 1. doi: 10.1186/2046-4053-4-1 . PMC 4320440 . PMID 25554246.
- ↑ Vale, C; Stewart, L; Tierney, J (2005). "Trends in UK Cancer Trials: Results from the UK Coordinating Committee for Cancer Research National Register of Cancer Trials". Br J Cancer. 92 (5): 811–814. doi:10.1038/sj.bjc.6602425. PMC 2361907 . PMID 15756251.
- ↑ "PROSPERO - International prospective register of systematic reviews". The University of York Centre for Reviews and Dissemination. University of York. 29 April 2015. Retrieved 23 May 2015.
- ↑ Booth, A; Stewart, L (2013). "Trusting researchers to use open trial registers such as PROSPERO responsibly". BMJ. 347 (f5870): f5870. doi:10.1136/bmj.f5870. PMID 24088555. S2CID 206900112 . Retrieved 25 May 2015.
- ↑ Stewart, L; Moher, D; Shekelle, P (2012). "Why prospective registration of systematic reviews makes sense". Syst Rev. 1 (7): 7. doi: 10.1186/2046-4053-1-7 . PMC 3369816 . PMID 22588008.
- ↑ Booth, A; Clarke, M; Dooley, G; Ghersi, D; Moher, D; Petticrew, M; Stewart, L (2012). "The nuts and bolts of PROSPERO: an international prospective register of systematic reviews". Syst Rev. 1 (2): 2. doi: 10.1186/2046-4053-1-2 . PMC 3348673 . PMID 22587842.
- ↑ Booth, A; Clarke, M; Ghersi, D; Moher, D; Petticrew, M; Stewart, L (2011). "Establishing a minimum dataset for prospective registration of systematic reviews: an international consultation". PLOS ONE. 6 (11): e27319. Bibcode:2011PLoSO...627319B. doi: 10.1371/journal.pone.0027319 . PMC 3217945 . PMID 22110625 . Retrieved 25 May 2015.
- ↑ Booth, A; Clarke, M; Ghersi, D; Moher, D; Petticrew, M; Stewart, L (2011). "An international registry of systematic-review protocols" (PDF). Lancet. 377 (9760): 108–109. doi:10.1016/S0140-6736(10)60903-8. PMID 20630580. S2CID 30588870 . Retrieved 25 May 2015.
- ↑ "YODA Project". Center for Outcomes Research & Evaluation (CORE). Yale School of Medicine. 2015. Retrieved 23 May 2015.
- ↑ Rodgers, MA; Brown, JV; Heirs, MK; Higgins, JP; Mannion, RJ; Simmonds, MC; Stewart, LA (2013). "Reporting of industry funded study outcome data: comparison of confidential and published data on the safety and effectiveness of rhBMP-2 for spinal fusion". BMJ. 346 (f3981): f3981. doi:10.1136/bmj.f3981. PMC 3687771 . PMID 23788229.
- ↑ "Society for Research Synthesis Methodology - Home". Society for Research Synthesis Methodology. 2015. Retrieved 23 May 2015.