
Heinrich Hermann Robert Koch was a German physician and microbiologist. As the discoverer of the specific causative agents of deadly infectious diseases including tuberculosis, cholera, and anthrax, he is regarded as one of the main founders of modern bacteriology. As such he is popularly nicknamed the father of microbiology, and as the father of medical bacteriology. His discovery of the anthrax bacterium in 1876 is considered as the birth of modern bacteriology. His discoveries directly provided proofs for the germ theory of diseases, and the scientific basis of public health.

Louis Pasteur was a French chemist and microbiologist renowned for his discoveries of the principles of vaccination, microbial fermentation, and pasteurization. His research in chemistry led to remarkable breakthroughs in the understanding of the causes and preventions of diseases, which laid down the foundations of hygiene, public health and much of modern medicine. His works are credited to saving millions of lives through the developments of vaccines for rabies and anthrax. He is regarded as one of the founders of modern bacteriology and has been honoured as the "father of bacteriology" and as the "father of microbiology".

A microorganism, or microbe, is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells.
Serendipity is an unplanned fortunate discovery. Serendipity is a common occurrence throughout the history of product invention and scientific discovery. Serendipity is also seen as a potential design principle for online activities that would present a wide array of information and viewpoints, rather than just re-enforcing a user's opinion.

Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. It is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic chemical synthesis. Tartaric acid is an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics, and is a dihydroxyl derivative of succinic acid.

Ilya Ilyich Mechnikov was a Russian zoologist of Romanian nobility ancestry and Ukrainian Jewish origin best known for his pioneering research in immunology. He and Paul Ehrlich were jointly awarded the 1908 Nobel Prize in Physiology or Medicine "in recognition of their work on immunity". He was born, lived and worked for many years on the territory of the Russian Empire. Given this complex heritage, four different nations and peoples justifiably lay claim to Mechnikov.

In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable, much as one's left and right hands are mirror images of each other that cannot appear identical simply by reorientation. A single chiral atom or similar structural feature in a compound causes that compound to have two possible structures which are non-superposable, each a mirror image of the other. Each member of the pair is termed an enantiomorph ; the structural property is termed enantiomerism. The presence of multiple chiral features in a given compound increases the number of geometric forms possible, though there may still be some perfect-mirror-image pairs.
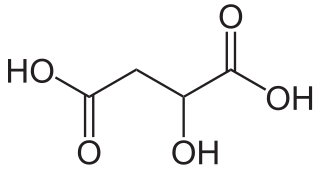
Malic acid is an organic compound with the molecular formula C4H6O5. It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms, though only the L-isomer exists naturally. The salts and esters of malic acid are known as malates. The malate anion is an intermediate in the citric acid cycle.

Félix d'Hérelle was a French microbiologist. He was co-discoverer of bacteriophages and experimented with the possibility of phage therapy. D'Herelle has also been credited for his contributions to the larger concept of applied microbiology.
In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic form. Half of the optically active substance becomes its mirror image (enantiomer) referred as racemic mixtures. If the racemization results in a mixture where the D and L enantiomers are present in equal quantities, the resulting sample is described as a racemic mixture or a racemate. Racemization can proceed through a number of different mechanisms, and it has particular significance in pharmacology as different enantiomers may have different pharmaceutical effects.

The Pasteur Institute is a French non-profit private foundation dedicated to the study of biology, micro-organisms, diseases, and vaccines. It is named after Louis Pasteur, who invented pasteurization and vaccines for anthrax and rabies. The institute was founded on June 4, 1887, and inaugurated on November 14, 1888.
Homochirality is a uniformity of chirality, or handedness. Objects are chiral when they cannot be superposed on their mirror images. For example, the left and right hands of a human are approximately mirror images of each other but are not their own mirror images, so they are chiral. In biology, 19 of the 20 natural amino acids are homochiral, being L-chiral (left-handed), while sugars are D-chiral (right-handed). Homochirality can also refer to enantiopure substances in which all the constituents are the same enantiomer, but some sources discourage this use of the term.

The history of penicillin follows a number of observations and discoveries of apparent evidence of antibiotic activity of the mould Penicillium that led to the development of penicillins that became the most widely used antibiotics. Following the identification of Penicillium rubens as the source of the compound in 1928 and with the production of pure compound in 1942, penicillin became the first naturally derived antibiotic. There are anecdotes about ancient societies using moulds to treat infections, and in the following centuries many people observed the inhibition of bacterial growth by various moulds. However, it is unknown if the species involved were Penicillium species or if the antimicrobial substances produced were penicillin.
The following are notable events in the Timeline of immunology:

Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food production, it may more broadly refer to any process in which the activity of microorganisms brings about a desirable change to a foodstuff or beverage. The science of fermentation is known as zymology.
Chiral resolution, or enantiomeric resolution, is a process in stereochemistry for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active compounds, including drugs. Another term with the same meaning is optical resolution.

Microbiology is the scientific study of microorganisms, those being unicellular, multicellular, or acellular. Microbiology encompasses numerous sub-disciplines including virology, bacteriology, protistology, mycology, immunology and parasitology.
The French Louis Pasteur (1822–1895) and German Robert Koch (1843–1910) are the two greatest figures in medical microbiology and in establishing acceptance of the germ theory of disease. In 1882, fueled by national rivalry and a language barrier, the tension between Pasteur and the younger Koch erupted into an acute conflict.
The role of chance, or "luck", in science comprises all ways in which unexpected discoveries are made.

Serendipity: Accidental Discoveries in Science is a 1989 popular science book by the chemist Royston M. Roberts. It received positive and mixed reviews; critics found it informative and entertaining, though some indicated that it contained errors.















