Pests and parasites
Varroa mites
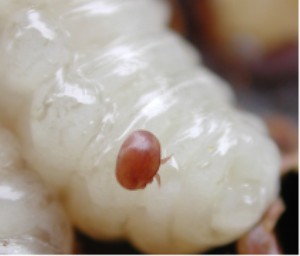
Varroa destructor and V. jacobsoni are parasitic mites that feed on the fat bodies of adult, pupal and larval bees. When the hive is very heavily infested, Varroa mites can be seen with the naked eye as a small red or brown spot on the bee's thorax. Varroa mites are carriers for many viruses that are damaging to bees. For example, bees infected during their development will often have visibly deformed wings.[ citation needed ]
Varroa mites have led to the virtual elimination of feral bee colonies in many areas, and are a major problem for kept bees in apiaries. Some feral populations are now recovering—it appears they have been naturally selected for Varroa resistance.[ citation needed ]
Varroa mites were first discovered in Southeast Asia in about 1904, but are now present on all continents except Australia. They were discovered in the United States in 1987, in New Zealand in 2000, and in Devon, United Kingdom in 1992.[ citation needed ]
To the untrained eye, these mites are generally not a very noticeable problem for a strongly growing hive- as the bees may appear strong in number, and may even be very effective at foraging. However, the mite reproduction cycle occurs inside the capped pupae, and the mite population can surge as a result of colony growth. Careful observation of a colony can help identify signs of disease often spread by mites. When the hive population growth is reduced in preparation for winter or due to poor late summer forage, the mite population growth can overtake that of the bees and can then destroy the hive. It has been observed diseased colonies may slowly die off and be unable to survive through winter even when adequate food stores are present. Often a colony will simply abscond (leave as in a swarm, but leaving no population behind) under such conditions.[ citation needed ]
Varroa in combination with viral vectors and bacteria have been theoretically implicated in colony collapse disorder.[ citation needed ]
It is known that thymol, a compound produced by thyme, naturally occurring in thyme honey, is a treatment for Varroa, though it may cause bee mortality at high concentrations. [1] Provisioning active colonies with crops of thyme may provide the colony with a non-interventional chemical defense against Varroa.[ citation needed ]
Treatment
A variety of chemical and mechanical treatments are used to attempt to control Varroa mites.[ citation needed ]
- "Hard" chemicals
"Hard" chemical treatments include amitraz (marketed as "Apivar" [2] ), fluvalinate (marketed as "Apistan"), coumaphos (marketed as CheckMite), flumethrin (marketed as "Bayvarol" and "Polyvar Yellow").
- "Soft" chemicals
"Soft" chemical treatments include thymol (marketed as "ApiLife-VAR [3] " and "Apiguard"), sucrose octanoate esters (marketed as "Sucrocide"), oxalic acid (marked as "Api-bioxal [4] ") and formic acid (sold in liquid form or in gel strips as Mite Away Quick Strips and Formic Pro, [5] but also used in other formulations).
According to the U.S. Environmental Protection Agency, when used in beehives as directed, chemical treatments kill a large proportion of the mites while not substantially disrupting bee behavior or life span. Use of chemical controls is generally regulated and varies from country to country. With few exceptions, they are not intended for use during production of marketable honey. [6]
- "Mechanical" treatments
Common mechanical controls generally rely on disruption of some aspect of the mites' lifecycle. These controls are generally intended not to eliminate all mites, but merely to maintain the infestation at a level which the colony can tolerate. Examples of mechanical controls include drone brood sacrifice (Varroa mites are preferentially attracted to the drone brood), powdered sugar dusting (which encourages cleaning behavior and dislodges some mites), screened bottom boards (so any dislodged mites fall through the bottom and away from the colony), brood interruption and, perhaps, downsizing of the brood cell size.[ citation needed ]
Acarine (tracheal) mites
Acarapis woodi is a parasitic mite that infests the trachea that lead from the first pair of thoracic spiracles. An unidentified bee illness was first reported on the Isle of Wight in England in 1904, becoming known as the 'Isle of Wight disease' (IoWD), which was initially thought to be caused by Acarapis woodi when it was identified in 1921 by Rennie. The IoWD disease quickly spread to the rest of Great Britain and Ireland, dealing a devastating blow to British and Irish beekeeping, being claimed as having wiped out the indigenous bee population of the British Isles. In 1991 Bailey and Ball stated "The final opinion of Rennie (1923), a co-discoverer of Acarapis woodi, who had much experience with bees said to have the Isle of Wight Disease, was that under the original and now quite properly discarded designation 'Isle of Wight Disease' were included several maladies having analogous superficial symptoms", [7] the authors came to the firm conclusion that the IoWD was not caused by acarine (Acarapis woodi) mites solely, but primarily by chronic bee paralysis virus (CBPV), even though Acarapis woodi was always found to be present within the hive whenever CBPV symptoms were observed. Brother Adam at Buckfast Abbey developed a resistant bee breed known as the Buckfast bee, which is now available worldwide.[ citation needed ]
Diagnosis for tracheal mites generally involves the dissection and microscopic examination of a sample of bees from the hive.[ citation needed ]
Acarapis woodi are believed to have entered the U.S. in 1984, from Mexico.[ citation needed ]
Mature female acarine mites leave the bee's airway and climb out on a hair of the bee, where they wait until they can transfer to a young bee. Once on the new bee, they move into the airways and begin laying eggs.[ citation needed ]
Treatment
Acarine mites are commonly controlled with grease patties (typically made from one part vegetable shortening mixed with three to four parts powdered sugar) placed on the top bars of the hive. The bees come to eat the sugar and pick up traces of shortening, which disrupts the mite's ability to identify a young bee. Some of the mites waiting to transfer to a new host remain on the original host. Others transfer to a random bee—a proportion of which will die of other causes before the mite can reproduce.[ citation needed ]
Menthol, either allowed to vaporize from crystal form or mixed into the grease patties, is also often used to treat acarine mites.[ citation needed ]
Nosema disease
Nosema apis is a microsporidian that invades the intestinal tracts of adult bees and causes Nosema disease, also known as nosemosis. [8] [9] Nosema infection is also associated with black queen cell virus. It spreads via fecal to oral matter to infect bees. [10] [11] [12] It is normally only a problem when the bees cannot leave the hive to eliminate waste (for example, during an extended cold spell in winter or when the hives are enclosed in a wintering barn). When the bees are unable to void (cleansing flights), they can develop dysentery. [13] [14]
Nosema disease is treated by increasing the ventilation through the hive. Some beekeepers treat hives with agents such as fumagillin. [15]
Nosemosis can also be prevented or minimized by removing much of the honey from the beehive, then feeding the bees on sugar water in the late fall. Sugar water made from refined sugar has lower ash content than flower nectar, reducing the risk of dysentery. Refined sugar, however, contains fewer nutrients than natural honey, which causes some controversy among beekeepers.[ citation needed ]
In 1996, a similar type of organism to N. apis was discovered on the Asian honey bee Apis cerana and subsequently named N. ceranae . This parasite apparently also infects the western honey bee. [16]
Exposure to corn pollen containing genes for Bacillus thuringiensis (Bt) production may weaken the bees' defense against Nosema. [17] In relation to feeding a group of bees with Bt corn pollen and a control group with non-Bt corn pollen: "in the first year, the bee colonies happened to be infested with parasites (microsporidia). This infestation led to a reduction in the number of bees and subsequently to reduced broods in the Bt-fed colonies, as well as in the colonies fed on Bt toxin-free pollen. The trial was then discontinued at an early stage. This effect was significantly more marked in the Bt-fed colonies. (The significant differences indicate an interaction of toxin and pathogen on the epithelial cells of the honeybee intestine. The underlying mechanism which causes this effect is unknown.)"[ citation needed ]
This study should be interpreted with caution given that no repetition of the experiment nor any attempt to find confounding factors was made. In addition, Bt toxin and transgenic Bt pollen showed no acute toxicity to any of the life stages of the bees examined, even when the Bt toxin was fed at concentrations 100 times that found in transgenic Bt pollen from maize.[ citation needed ]
Nosema disease is very common when bees get into winter clusters, as they spend an extensive time in their hives as they keep together for warmth and have little to no opportunities to eliminate waste.
Small hive beetle

Aethina tumida is a small, dark-colored beetle that lives in beehives. Originally from Africa, the first discovery of small hive beetles in the Western Hemisphere was made in St. Lucie County, Florida, in 1998. The next year, a specimen that had been collected from Charleston, South Carolina, in 1996 was identified, and is believed to be the index case for the United States. [18] By December 1999, small hive beetles were reported in Iowa, Maine, Massachusetts, Minnesota, New Jersey, Ohio, Pennsylvania, Texas, and Wisconsin, and it was found in California by 2006.[ citation needed ]
The lifecycle of this beetle includes pupation in the ground outside of the hive. Controls to prevent ants from climbing into the hive are believed to also be effective against the hive beetle. Several beekeepers are experimenting with the use of diatomaceous earth around the hive as a way to disrupt the beetle's lifecycle. The diatoms abrade the insects' surfaces, causing them to dehydrate and die.[ citation needed ]
Treatment
Several pesticides are currently used against the small hive beetle. The chemical fipronil (marketed as Combat Roach Gel [19] ) is commonly applied inside the corrugations of a piece of cardboard. Standard corrugations are large enough that a small hive beetle can enter the cardboard through the end, but small enough that honey bees cannot enter (thus are kept away from the pesticide). Alternative controls such as oil-based top-bar traps are also available, but they have had very little commercial success.[ citation needed ]
Wax moths

Galleria mellonella (greater wax moths) do not attack the bees directly, but feed on the shed exoskeletons of bee larvae and pollen that is found in dark brood comb, which was used by the bees to hold the developing bees. Their full development to adults requires access to used brood comb or brood cell cleanings—these contain protein essential for the larval development, in the form of brood cocoons. The destruction of the comb will spill or contaminate stored honey and may kill bee larvae.[ citation needed ]
When honey supers are stored for the winter in a mild climate, or in heated storage, the wax moth larvae can destroy portions of the comb, though they will not fully develop. Damaged comb may be scraped out and replaced by the bees. Wax moth larvae and eggs are killed by freezing, so storage in unheated sheds or barns in higher latitudes is the only control necessary.[ citation needed ]
Because wax moths cannot survive a cold winter, they are usually not a problem for beekeepers in the northern U.S. or Canada, unless they survive winter in heated storage, or are brought from the south by purchase or migration of beekeepers. They thrive and spread most rapidly with temperatures above 30 °C (90 °F), so some areas with only occasional days that are hot rarely have a problem with wax moths, unless the colony is already weak due to stress from other factors.[ citation needed ]
Control and treatment
A strong hive generally needs no treatment to control wax moths; the bees themselves kill and clean out the moth larvae and webs. Wax moth larvae may fully develop in cell cleanings when such cleanings accumulate thickly where they are not accessible to the bees.[ citation needed ]
Wax moth development in comb is generally not a problem with top bar hives, as unused combs are usually left in the hive during the winter. Since this type of hive is not used in severe wintering conditions, the bees are able to patrol and inspect the unused comb.[ citation needed ]
Wax moths can be controlled in stored comb by application of the aizawai variety of B. thuringiensis spores by spraying. It is a very effective biological control and has an excellent safety record.[ citation needed ]
Wax moths can be controlled chemically with paradichlorobenzene (moth crystals or urinal disks). If chemical methods are used, the combs must be well-aired for several days before use. The use of naphthalene (mothballs) is discouraged because it accumulates in the wax, which can kill bees or contaminate honey stores.
Control of wax moths by other means includes the freezing of the comb for a few hours. [20] Langstroth found that placing a spider, such as a daddy-long-legs, with stored combs controlled wax moth and eliminate the need for hash chemicals. [21] This has been confirmed more recently by others, such as Bergqvist. [22]
Tropilaelaps
Tropilaelaps mercedesae and T. clareae are considered serious threats to honeybees. Although they are not currently found outside Asia, these mites have the potential to inflict serious damage to colonies due to their rapid reproduction inside the hive. [23]













