A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.

An endospore is a dormant, tough, and non-reproductive structure produced by some bacteria in the phylum Bacillota. The name "endospore" is suggestive of a spore or seed-like form, but it is not a true spore. It is a stripped-down, dormant form to which the bacterium can reduce itself. Endospore formation is usually triggered by a lack of nutrients, and usually occurs in gram-positive bacteria. In endospore formation, the bacterium divides within its cell wall, and one side then engulfs the other. Endospores enable bacteria to lie dormant for extended periods, even centuries. There are many reports of spores remaining viable over 10,000 years, and revival of spores millions of years old has been claimed. There is one report of viable spores of Bacillus marismortui in salt crystals approximately 25 million years old. When the environment becomes more favorable, the endospore can reactivate itself into a vegetative state. Most types of bacteria cannot change to the endospore form. Examples of bacterial species that can form endospores include Bacillus cereus, Bacillus anthracis, Bacillus thuringiensis, Clostridium botulinum, and Clostridium tetani. Endospore formation is not found among Archaea.

Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines; at one time acridine dyes were popular, but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound.

Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.

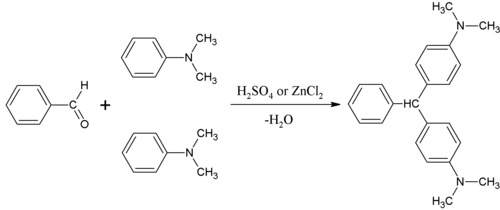
N,N-Dimethylaniline (DMA) is an organic chemical compound, a substituted derivative of aniline. It consists of a tertiary amine, featuring dimethylamino group attached to a phenyl group. This oily liquid is colourless when pure, but commercial samples are often yellow. It is an important precursor to dyes such as crystal violet.

In molecular biology and biotechnology, a fluorescent tag, also known as a fluorescent label or fluorescent probe, is a molecule that is attached chemically to aid in the detection of a biomolecule such as a protein, antibody, or amino acid. Generally, fluorescent tagging, or labeling, uses a reactive derivative of a fluorescent molecule known as a fluorophore. The fluorophore selectively binds to a specific region or functional group on the target molecule and can be attached chemically or biologically. Various labeling techniques such as enzymatic labeling, protein labeling, and genetic labeling are widely utilized. Ethidium bromide, fluorescein and green fluorescent protein are common tags. The most commonly labelled molecules are antibodies, proteins, amino acids and peptides which are then used as specific probes for detection of a particular target.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.
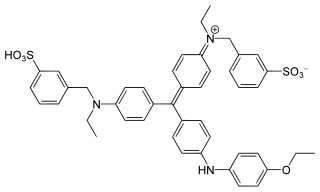
Coomassie brilliant blue is the name of two similar triphenylmethane dyes that were developed for use in the textile industry but are now commonly used for staining proteins in analytical biochemistry. Coomassie brilliant blue G-250 differs from Coomassie brilliant blue R-250 by the addition of two methyl groups. The name "Coomassie" is a registered trademark of Imperial Chemical Industries.

Astaxanthin is a keto-carotenoid within a group of chemical compounds known as terpenes. Astaxanthin is a metabolite of zeaxanthin and canthaxanthin, containing both hydroxyl and ketone functional groups. It is a lipid-soluble pigment with red coloring properties, which result from the extended chain of conjugated double bonds at the center of the compound. The presence of the hydroxyl functional groups and the hydrophobic hydrocarbons render the molecule amphiphilic.

Rhodamine is a family of related dyes, a subset of the triarylmethane dyes. They are derivatives of xanthene. Important members of the rhodamine family are Rhodamine 6G, Rhodamine 123, and Rhodamine B. They are mainly used to dye paper and inks, but they lack the lightfastness for fabric dyeing.

Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display fluorescence. A trityl group in organic chemistry is a triphenylmethyl group Ph3C, e.g. triphenylmethyl chloride (trityl chloride) and the triphenylmethyl radical (trityl radical).

New fuchsine is an organic compound with the formula [(H2N(CH3)C6H3)3C]Cl. It is a green-colored solid that is used as a dye of the triarylmethane class. It is one of the four components of basic fuchsine, and one of the two that are available as single dyes. The other is pararosaniline. It is prepared by condensation of ortho-toluidine with formaldehyde. This process initially gives the benzhydrol 4,4'-bis(dimethylamino)benzhydrol, which is further condensed to give the leuco (colorless) tertiary alcohol [(H2N(CH3)C6H3)3COH, which is oxidized in acid to give the dye.

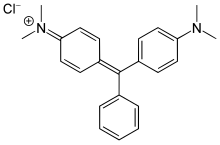
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.

An acid dye is a dye that is typically applied to a textile at low pH. They are mainly used to dye wool, not cotton fabrics. Some acid dyes are used as food colorants, and some can also be used to stain organelles in the medical field.

Brilliant blue FCF is a synthetic organic compound used primarily as a blue colorant for processed foods, medications, dietary supplements, and cosmetics. It is classified as a triarylmethane dye and is known under various names, such as FD&C Blue No. 1 or acid blue 9. It is denoted by E number E133 and has a color index of 42090. It has the appearance of a blue powder and is soluble in water and glycerol, with a maximum absorption at about 628 nanometers. It is one of the oldest FDA-approved color additives and is generally considered nontoxic and safe.

Fast Green FCF, also called Food green 3, FD&C Green No. 3, Green 1724, Solid Green FCF, and C.I. 42053, is a turquoise triarylmethane food dye. Its E number is E143.
Triarylmethane dyes are synthetic organic compounds containing triphenylmethane backbones. As dyes, these compounds are intensely colored. They are produced industrially as dyes.

Brilliant green is one of the triarylmethane dyes. It is closely related to malachite green.

Endospore staining is a technique used in bacteriology to identify the presence of endospores in a bacterial sample. Within bacteria, endospores are protective structures used to survive extreme conditions, including high temperatures making them highly resistant to chemicals. Endospores contain little or no ATP which indicates how dormant they can be. Endospores contain a tough outer coating made up of keratin which protects them from nucleic DNA as well as other adaptations. Endospores are able to regerminate into vegetative cells, which provides a protective nature that makes them difficult to stain using normal techniques such as simple staining and gram staining. Special techniques for endospore staining include the Schaeffer–Fulton stain and the Moeller stain.
Antimicrobials destroy bacteria, viruses, fungi, algae, and other microbes. The cells of bacteria (prokaryotes), such as salmonella, differ from those of higher-level organisms (eukaryotes), such as fish. Antibiotics are chemicals designed to either kill or inhibit the growth of pathogenic bacteria while exploiting the differences between prokaryotes and eukaryotes in order to make them relatively harmless in higher-level organisms. Antibiotics are constructed to act in one of three ways: by disrupting cell membranes of bacteria, by impeding DNA or protein synthesis, or by hampering the activity of certain enzymes unique to bacteria.