Related Research Articles

The Morse potential, named after physicist Philip M. Morse, is a convenient interatomic interaction model for the potential energy of a diatomic molecule. It is a better approximation for the vibrational structure of the molecule than the quantum harmonic oscillator because it explicitly includes the effects of bond breaking, such as the existence of unbound states. It also accounts for the anharmonicity of real bonds and the non-zero transition probability for overtone and combination bands. The Morse potential can also be used to model other interactions such as the interaction between an atom and a surface. Due to its simplicity, it is not used in modern spectroscopy. However, its mathematical form inspired the MLR (Morse/Long-range) potential, which is the most popular potential energy function used for fitting spectroscopic data.
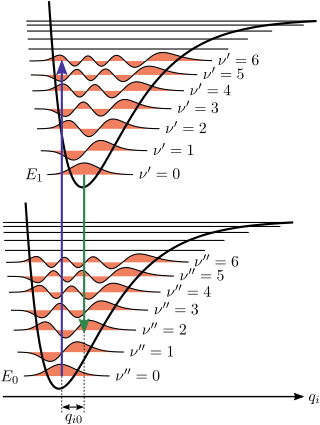
The Franck–Condon principle is a rule in spectroscopy and quantum chemistry that explains the intensity of vibronic transitions. The principle states that during an electronic transition, a change from one vibrational energy level to another will be more likely to happen if the two vibrational wave functions overlap more significantly.

Nicholas Charles Handy was a British theoretical chemist. He retired as Professor of quantum chemistry at the University of Cambridge in September 2004.
Rydberg ionization spectroscopy is a spectroscopy technique in which multiple photons are absorbed by an atom causing the removal of an electron to form an ion.

Spartan is a molecular modelling and computational chemistry application from Wavefunction. It contains code for molecular mechanics, semi-empirical methods, ab initio models, density functional models, post-Hartree–Fock models, and thermochemical recipes including G3(MP2) and T1. Quantum chemistry calculations in Spartan are powered by Q-Chem.
Ab initio quantum chemistry methods are computational chemistry methods based on quantum chemistry. The term ab initio was first used in quantum chemistry by Robert Parr and coworkers, including David Craig in a semiempirical study on the excited states of benzene. The background is described by Parr. Ab initio means "from first principles" or "from the beginning", implying that the only inputs into an ab initio calculation are physical constants. Ab initio quantum chemistry methods attempt to solve the electronic Schrödinger equation given the positions of the nuclei and the number of electrons in order to yield useful information such as electron densities, energies and other properties of the system. The ability to run these calculations has enabled theoretical chemists to solve a range of problems and their importance is highlighted by the awarding of the Nobel prize to John Pople and Walter Kohn.

Amyand David Buckingham born in Pymble, Sydney, New South Wales, Australia was a chemist, with primary expertise in chemical physics.
Vibrational circular dichroism (VCD) is a spectroscopic technique which detects differences in attenuation of left and right circularly polarized light passing through a sample. It is the extension of circular dichroism spectroscopy into the infrared and near infrared ranges.

Eric Johnson "Rick" Heller is the Abbott and James Lawrence Professor of Chemistry and Professor of Physics at Harvard University. Heller is known for his work on time-dependent quantum mechanics, and also for producing digital art based on the results of his numerical calculations.

In chemistry, a water cluster is a discrete hydrogen bonded assembly or cluster of molecules of water. Many such clusters have been predicted by theoretical models (in silico), and some have been detected experimentally in various contexts such as ice, bulk liquid water, in the gas phase, in dilute mixtures with non-polar solvents, and as water of hydration in crystal lattices. The simplest example is the water dimer (H2O)2.
Rydberg matter is an exotic phase of matter formed by Rydberg atoms; it was predicted around 1980 by É. A. Manykin, M. I. Ozhovan and P. P. Poluéktov. It has been formed from various elements like caesium, potassium, hydrogen and nitrogen; studies have been conducted on theoretical possibilities like sodium, beryllium, magnesium and calcium. It has been suggested to be a material that diffuse interstellar bands may arise from. Circular Rydberg states, where the outermost electron is found in a planar circular orbit, are the most long-lived, with lifetimes of up to several hours, and are the most common.
Giacinto Scoles is a European and North American chemist and physicist who is best known for his pioneering development of molecular beam methods for the study of weak van der Waals forces between atoms, molecules, and surfaces. He developed the cryogenic bolometer as a universal detector of atomic and molecule beams that not only can detect a small flux of molecules, but also responds to the internal energy of the molecules. This is the basis for the optothermal spectroscopy technique which Scoles and others have used to obtain very high signal-to noise and high resolution ro-vibrational spectra.

HCNH+, also known as protonated hydrogen cyanide, is a molecular ion of astrophysical interest. It also exists in the condensed state when formed by superacids.

CP2K is a freely available (GPL) quantum chemistry and solid state physics program package, written in Fortran 2008, to perform atomistic simulations of solid state, liquid, molecular, periodic, material, crystal, and biological systems. It provides a general framework for different methods: density functional theory (DFT) using a mixed Gaussian and plane waves approach (GPW) via LDA, GGA, MP2, or RPA levels of theory, classical pair and many-body potentials, semi-empirical and tight-binding Hamiltonians, as well as Quantum Mechanics/Molecular Mechanics (QM/MM) hybrid schemes relying on the Gaussian Expansion of the Electrostatic Potential (GEEP). The Gaussian and Augmented Plane Waves method (GAPW) as an extension of the GPW method allows for all-electron calculations. CP2K can do simulations of molecular dynamics, metadynamics, Monte Carlo, Ehrenfest dynamics, vibrational analysis, core level spectroscopy, energy minimization, and transition state optimization using NEB or dimer method.
Triatomic hydrogen or H3 is an unstable triatomic molecule containing only hydrogen. Since this molecule contains only three atoms of hydrogen it is the simplest triatomic molecule and it is relatively simple to numerically solve the quantum mechanics description of the particles. Being unstable the molecule breaks up in under a millionth of a second. Its fleeting lifetime makes it rare, but it is quite commonly formed and destroyed in the universe thanks to the commonness of the trihydrogen cation. The infrared spectrum of H3 due to vibration and rotation is very similar to that of the ion, H+
3. In the early universe this ability to emit infrared light allowed the primordial hydrogen and helium gas to cool down so as to form stars.
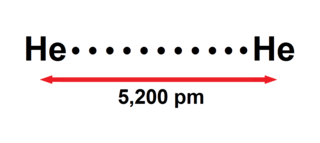
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak that it will break if the molecule rotates, or vibrates too much. It can only exist at very low cryogenic temperatures.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.

Martin Quack is a German physical chemist and spectroscopist; he is a professor at ETH Zürich.

Bretislav Friedrich is a Research Group leader at the Department of Molecular Physics, Fritz-Haber-Institut der Max-Planck-Gesellschaft and Honorarprofessor at the Technische Universität in Berlin, Germany. He is globally recognized for his pioneering research surrounding interaction of molecules with and in electric, magnetic, and optical fields as well as on cold molecules. He was admitted to the Learned Society of the Czech Republic in 2011.

Dirubidium is a molecular substance containing two atoms of rubidium found in rubidium vapour. Dirubidium has two active valence electrons. It is studied both in theory and with experiment. The rubidium trimer has also been observed.
References
- ↑ "Chemistry Tree - Mark S. Child Details".
- ↑ Coveney, Peter V (1985). Semiclassical methods in scattering and spectroscopy (DPhil thesis). University of Oxford.
- ↑ CHILD, Prof. Mark Sheard, Who's Who 2014, A & C Black, 2014; online edn, Oxford University Press, 2014
- ↑ "Professor Mark Child FRS (1947-1955)". Old Pocklingtonians. 16 June 2021. Retrieved 17 January 2023.
- ↑ Mark Child (1991). Semiclassical mechanics with molecular applications. Oxford: Clarendon Press. ISBN 978-0-19-855654-1.
- ↑ Mark Child (1996). Molecular collision theory. New York: Dover Publications. ISBN 978-0-486-69437-5.
- ↑ Mark Child (2011). Theory of Molecular Rydberg States (Cambridge Molecular Science). Cambridge, UK: Cambridge University Press. ISBN 978-0-521-76995-2.
- ↑ Child, M. S.; Jungen, C. (1990). "Quantum defect theory for asymmetric tops: Application to the Rydberg spectrum of H2O". The Journal of Chemical Physics. 93 (11): 7756. Bibcode:1990JChPh..93.7756C. doi:10.1063/1.459355.
- ↑ Child, M. S. (1997). "The Rydberg states of H2O". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 355 (1729): 1623–1636. Bibcode:1997RSPTA.355.1623C. doi:10.1098/rsta.1997.0080. S2CID 93739122.
- ↑ Hiyama, M.; Child, M. S. (2002). "Ab initio R-matrix/multichannel quantum defect theory study of nitric oxide". Journal of Physics B: Atomic, Molecular and Optical Physics. 35 (5): 1337. Bibcode:2002JPhB...35.1337H. doi:10.1088/0953-4075/35/5/316. S2CID 250830242.
- ↑ Child, M. S.; Weston, T.; Tennyson, J. (1999). "Quantum monodromy in the spectrum of H2O and other systems: New insight into the level structure of quasi-linear molecules". Molecular Physics. 96 (3): 371. Bibcode:1999MolPh..96..371C. CiteSeerX 10.1.1.324.1149 . doi:10.1080/00268979909482971.
- ↑ Jacobson, M. P.; Child, M. S. (2001). "Spectroscopic signatures of bond-breaking internal rotation. II. Rotation-vibration level structure and quantum monodromy in HCP". The Journal of Chemical Physics. 114 (1): 262. Bibcode:2001JChPh.114..262J. doi:10.1063/1.1330746.
- ↑ Child, M. S. (2007). "Quantum Monodromyand Molecular Spectroscopy". Advances in Chemical Physics. Vol. 136. pp. 39–02. doi:10.1002/9780470175422.ch2. ISBN 9780470175422.
- ↑ Cooper, C. D.; Child, M. S. (2005). "Quantum level structures at a Fermi resonance with angular momentum: Classical periodic orbits, catastrophe maps and quantum monodromy". Physical Chemistry Chemical Physics. 7 (14): 2731–2739. Bibcode:2005PCCP....7.2731C. doi:10.1039/B502772C. PMID 16189587.