Related Research Articles
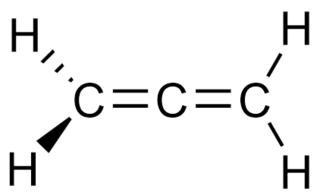
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which is itself also called allene. Compounds with an allene-type structure but with more than three carbon atoms are members of a larger class of compounds called cumulenes with X=C=Y bonding.

Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

Medicinal or pharmaceutical chemistry is a scientific discipline at the intersection of chemistry and pharmacy involved with designing and developing pharmaceutical drugs. Medicinal chemistry involves the identification, synthesis and development of new chemical entities suitable for therapeutic use. It also includes the study of existing drugs, their biological properties, and their quantitative structure-activity relationships (QSAR).
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: total synthesis, semisynthesis, and methodology.
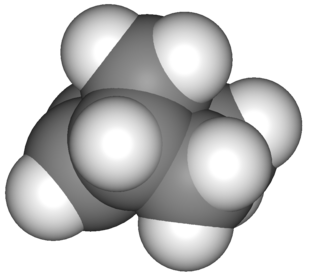
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carries a double bond which induces significant ring strain and significant reactivity.
Dynamic covalent chemistry (DCvC) is a synthetic strategy employed by chemists to make complex supramolecular assemblies from discrete molecular building blocks. DCvC has allowed access to complex assemblies such as covalent organic frameworks, molecular knots, polymers, and novel macrocycles. Not to be confused with dynamic combinatorial chemistry, DCvC concerns only covalent bonding interactions. As such, it only encompasses a subset of supramolecular chemistries.
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.

Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis, and reactions. Organotitanium compounds in organometallic chemistry contain carbon-titanium chemical bonds. They are reagents in organic chemistry and are involved in major industrial processes.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
The Cook–Heilbron thiazole synthesis highlights the formation of 5-aminothiazoles through the chemical reaction of α-aminonitriles or aminocynoacetates with dithioacids, carbon disulphide, carbon oxysulfide, or isothiocynates at room temperature and under mild or aqueous conditions. Variation of substituents at the 2nd and 4th position of the thiazole is introduced by selecting different combinations of starting reagents.

Organozirconium chemistry is the science of exploring the properties, structure, and reactivity of organozirconium compounds, which are organometallic compounds containing chemical bonds between carbon and zirconium. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.
In organic chemistry, a cross-coupling reaction is a reaction where two different fragments are joined. Cross-couplings are a subset of the more general coupling reactions. Often cross-coupling reactions require metal catalysts. One important reaction type is this:
David Milstein is an Israeli chemist studying homogeneous catalysis.
Organogold chemistry is the study of compounds containing gold–carbon bonds. They are studied in academic research, but have not received widespread use otherwise. The dominant oxidation states for organogold compounds are I with coordination number 2 and a linear molecular geometry and III with CN = 4 and a square planar molecular geometry.

The Catellani reaction was discovered by Marta Catellani and co-workers in 1997. The reaction uses aryl iodides to perform bi- or tri-functionalization, including C-H functionalization of the unsubstituted ortho position(s), followed a terminating cross-coupling reaction at the ipso position. This cross-coupling cascade reaction depends on the ortho-directing transient mediator, norbornene.

Mark Lautens, OC, is a Canadian organic chemist and is a University Professor at the University of Toronto.
In organic chemistry, the Fujiwara–Moritani reaction is a type of cross coupling reaction where an aromatic C-H bond is directly coupled to an olefinic C-H bond, generating a new C-C bond. This reaction is performed in the presence of a transition metal, typically palladium. The reaction was discovered by Yuzo Fujiwara and Ichiro Moritani in 1967. An external oxidant is required to this reaction to be run catalytically. Thus, this reaction can be classified as a C-H activation reaction, an oxidative Heck reaction, and a C-H olefination. Surprisingly, the Fujiwara–Moritani reaction was discovered before the Heck reaction.

The Mizoroki−Heck coupling of aryl halides and alkenes to form C(sp2)–C(sp2) bonds has become a staple transformation in organic synthesis, owing to its broad functional group compatibility and varied scope. In stark contrast, the palladium-catalyzed reductive Heck reaction has received considerably less attention, despite the fact that early reports of this reaction date back almost half a century. From the perspective of retrosynthetic logic, this transformation is highly enabling because it can forge alkyl–aryl linkages from widely available alkenes, rather than from the less accessible and/or more expensive alkyl halide or organometallic C(sp3) synthons that are needed in a classical aryl/alkyl cross-coupling.
Organotechnetium chemistry is the science of describing the physical properties, synthesis, and reactions of organotechnetium compounds, which are organometallic compounds containing carbon-to-technetium chemical bonds. The most common organotechnetium compounds are coordination complexes used as radiopharmaceutical imaging agents.
References
- ↑ "European Academy of Sciences - Marta Catellani". www.eurasc.org. Retrieved 2019-10-18.
- 1 2 3 "Prof. Dr. Marta Catellani « ISHC 2018" . Retrieved 2019-10-18.
- 1 2 3 Olson, Julie A.; Shea, Kevin M. (2011-05-17). "Critical Perspective: Named Reactions Discovered and Developed by Women". Accounts of Chemical Research. 44 (5): 311–321. doi:10.1021/ar100114m. ISSN 0001-4842. PMID 21417324.
- ↑ Liu, Ze-Shui; Gao, Qianwen; Cheng, Hong-Gang; Zhou, Qianghui (2018-10-17). "Alkylating Reagents Employed in Catellani-Type Reactions". Chemistry: A European Journal. 24 (58): 15461–15476. doi:10.1002/chem.201802818. ISSN 1521-3765. PMID 30016558.
- 1 2 Yamamoto, Y.; Murayama, T.; Jiang, J.; Yasui, T.; Shibuya, M. (2018-01-31). "The vinylogous Catellani reaction: a combined computational and experimental study". Chemical Science. 9 (5): 1191–1199. doi:10.1039/C7SC04265E. ISSN 2041-6539. PMC 5885779 . PMID 29675164.
- ↑ Martins, Andrew; Mariampillai, Brian; Lautens, Mark (2010). Synthesis in the Key of Catellani: Norbornene-Mediated ortho C-H Functionalization. Cha. Topics in Current Chemistry. Vol. 292. pp. 1–33. Bibcode:2010cha..book....1M. doi:10.1007/128_2009_13. ISBN 978-3-642-12355-9. PMID 21500401.
- ↑ Wheeler2019-03-18T08:06:00+00:00, Philip. "Creating carbon–carbon bonds via transition metal catalysis". Chemistry World. Retrieved 2020-04-26.
- 1 2 Weinstabl, Harald; Suhartono, Marcel; Qureshi, Zafar; Lautens, Mark (2013-05-10). "Total Synthesis of (+)-Linoxepin by Utilizing the Catellani Reaction". Angewandte Chemie International Edition in English. 52 (20): 5305–5308. doi:10.1002/anie.201302327. ISSN 1433-7851. PMC 3715096 . PMID 23592590.