Related Research Articles

In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle, transforming into an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in so-called positron emission. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron emission to be energetically possible, the energy release or Q value must be positive.
A chemical element is a chemical substance that cannot be broken down into other substances by chemical reactions. The basic particle that constitutes a chemical element is the atom. Chemical elements are identified by the number of protons in the nuclei of their atoms, known as the element's atomic number. For example, oxygen has an atomic number of 8, meaning that each oxygen atom has 8 protons in its nucleus. Two or more atoms of the same element can combine to form molecules, in contrast to chemical compounds or mixtures, which contain atoms of different elements. Atoms can be transformed into different elements in nuclear reactions, which change an atom's atomic number.

Promethium is a chemical element; it has symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of only two radioactive elements that are followed in the periodic table by elements with stable forms, the other being technetium. Chemically, promethium is a lanthanide. Promethium shows only one stable oxidation state of +3.

Stable nuclides are nuclides that are not radioactive and so do not spontaneously undergo radioactive decay. When such nuclides are referred to in relation to specific elements, they are usually termed stable isotopes.
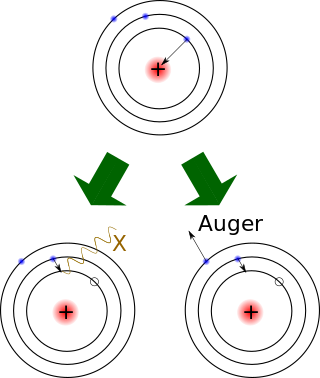
Electron capture is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton to a neutron and simultaneously causes the emission of an electron neutrino.

A nuclide is a class of atoms characterized by their number of protons, Z, their number of neutrons, N, and their nuclear energy state.

In nuclear physics, a magic number is a number of nucleons such that they are arranged into complete shells within the atomic nucleus. As a result, atomic nuclei with a 'magic' number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126.

The mass number (symbol A, from the German word: Atomgewicht, "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. Since protons and neutrons are both baryons, the mass number A is identical with the baryon number B of the nucleus (and also of the whole atom or ion). The mass number is different for each isotope of a given chemical element, and the difference between the mass number and the atomic number Z gives the number of neutrons (N) in the nucleus: N = A − Z.

In nuclear physics, double beta decay is a type of radioactive decay in which two neutrons are simultaneously transformed into two protons, or vice versa, inside an atomic nucleus. As in single beta decay, this process allows the atom to move closer to the optimal ratio of protons and neutrons. As a result of this transformation, the nucleus emits two detectable beta particles, which are electrons or positrons.
Lead (82Pb) has four observationally stable isotopes: 204Pb, 206Pb, 207Pb, 208Pb. Lead-204 is entirely a primordial nuclide and is not a radiogenic nuclide. The three isotopes lead-206, lead-207, and lead-208 represent the ends of three decay chains: the uranium series, the actinium series, and the thorium series, respectively; a fourth decay chain, the neptunium series, terminates with the thallium isotope 205Tl. The three series terminating in lead represent the decay chain products of long-lived primordial 238U, 235U, and 232Th. Each isotope also occurs, to some extent, as primordial isotopes that were made in supernovae, rather than radiogenically as daughter products. The fixed ratio of lead-204 to the primordial amounts of the other lead isotopes may be used as the baseline to estimate the extra amounts of radiogenic lead present in rocks as a result of decay from uranium and thorium.
Technetium (43Tc) is one of the two elements with Z < 83 that have no stable isotopes; the other such element is promethium. It is primarily artificial, with only trace quantities existing in nature produced by spontaneous fission or neutron capture by molybdenum. The first isotopes to be synthesized were 97Tc and 99Tc in 1936, the first artificial element to be produced. The most stable radioisotopes are 97Tc, 98Tc, and 99Tc.
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be synthesized and identified was 239Np in 1940, produced by bombarding 238
U
with neutrons to produce 239
U
, which then underwent beta decay to 239
Np
.
A table or chart of nuclides is a two-dimensional graph of isotopes of the elements, in which one axis represents the number of neutrons and the other represents the number of protons in the atomic nucleus. Each point plotted on the graph thus represents a nuclide of a known or hypothetical chemical element. This system of ordering nuclides can offer a greater insight into the characteristics of isotopes than the better-known periodic table, which shows only elements and not their isotopes. The chart of the nuclides is also known as the Segrè chart, after the Italian physicist Emilio Segrè.

The neutron number is the number of neutrons in a nuclide.

Isotopes are distinct nuclear species of the same chemical element. They have the same atomic number and position in the periodic table, but differ in nucleon numbers due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties.

In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the interstellar medium from which the solar system was formed, and were formed in, or after, the Big Bang, by nucleosynthesis in stars and supernovae followed by mass ejection, by cosmic ray spallation, and potentially from other processes. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present; 286 such nuclides are known.

Beta-decay stable isobars are the set of nuclides which cannot undergo beta decay, that is, the transformation of a neutron to a proton or a proton to a neutron within the nucleus. A subset of these nuclides are also stable with regards to double beta decay or theoretically higher simultaneous beta decay, as they have the lowest energy of all isobars with the same mass number.

Isobars are atoms (nuclides) of different chemical elements that have the same number of nucleons. Correspondingly, isobars differ in atomic number but have the same mass number. An example of a series of isobars is 40S, 40Cl, 40Ar, 40K, and 40Ca. While the nuclei of these nuclides all contain 40 nucleons, they contain varying numbers of protons and neutrons.

In nuclear physics, properties of a nucleus depend on evenness or oddness of its atomic number Z, neutron number N and, consequently, of their sum, the mass number A. Most importantly, oddness of both Z and N tends to lower the nuclear binding energy, making odd nuclei generally less stable. This effect is not only experimentally observed, but is included in the semi-empirical mass formula and explained by some other nuclear models, such as the nuclear shell model. This difference of nuclear binding energy between neighbouring nuclei, especially of odd-A isobars, has important consequences for beta decay.
References
- 1 2 3 4 Thyssen, Pieter; Binnemans, Koen; Shinohara, Hisanori; Saito, Yahachi; Gulay, Lubomir D.; Daszkiewicz, Marek; Yan, Chun-Hua; Yan, Zheng-Guan; Du, Ya-Ping (2011). Gschneider, Karl A. Jr.; Bünzli, Jean-Claude; Pecharsky, Vitalij K. (eds.). Handbook on the Physics and Chemistry of Rare Earths. Amsterdam, The Netherlands: Elsevier. p. 66. ISBN 978-0-444-53590-0 . Retrieved January 14, 2012.
- 1 2 Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, p. 84, ISBN 0-12-352651-5
- ↑ Wang, M.; Audi, G.; Kondev, F. G.; Huang, W. J.; Naimi, S.; Xu, X. (2017). "The AME2016 atomic mass evaluation (II). Tables, graphs, and references" (PDF). Chinese Physics C. 41 (3): 030003-1–030003-442. doi:10.1088/1674-1137/41/3/030003.
- ↑ K.S. Krane (1988). Introductory Nuclear Physics . John Wiley & Sons. p. 381. ISBN 978-0-471-80553-3.
- ↑ Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brookhaven National Laboratory. Retrieved 27 November 2012.