Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.

Entropy is a scientific concept, as well as a measurable physical property that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far ranging applications in chemistry and physics, biological systems and their relation to life, cosmology, economics, sociology, weather science and climate change, and information systems and the transmission of information in telecommunication.

Enthalpy is a property of a thermodynamic system, defined as the sum of the system's internal energy and the product of its pressure and volume. It is a convenient state function standardly used in many measurements in chemical, biological, and physical systems at a constant pressure. The pressure-volume term expresses the work required to establish the system's physical dimensions, i.e. to make room for it by displacing its surroundings. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it.
Statistical mechanics, one of the pillars of modern physics, describes how macroscopic observations are related to microscopic parameters that fluctuate around an average. It connects thermodynamic quantities to microscopic behavior, whereas, in classical thermodynamics, the only available option would be to measure and tabulate such quantities for various materials.

Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, radiation, and physical properties of matter. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.

Thermochemistry is the study of the heat energy which is associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the system's energy exchange with its surroundings. Thermochemistry is useful in predicting reactant and product quantities throughout the course of a given reaction. In combination with entropy determinations, it is also used to predict whether a reaction is spontaneous or non-spontaneous, favorable or unfavorable.

The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. Entropy predicts the direction of spontaneous processes, and determines whether they are irreversible or impossible, despite obeying the requirement of conservation of energy, which is established in the first law of thermodynamics. The second law may be formulated by the observation that the entropy of isolated systems left to spontaneous evolution cannot decrease, as they always arrive at a state of thermodynamic equilibrium, where the entropy is highest. If all processes in the system are reversible, the entropy is constant.

The first law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic processes, distinguishing two kinds of transfer of energy, as heat and as thermodynamic work, and relating them to a function of a body's state, called Internal energy.
Thermodynamic equilibrium is an axiomatic concept of thermodynamics. It is an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In thermodynamic equilibrium there are no net macroscopic flows of matter or of energy, either within a system or between systems.
A closed system is a physical system that does not allow transfer of matter in or out of the system, though, in different contexts, such as physics, chemistry or engineering, the transfer of energy is or is not allowed.
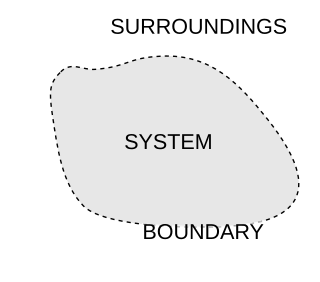
A thermodynamic system is a body of matter and/or radiation, confined in space by walls, with defined permeabilities, which separate it from its surroundings. The surroundings may include other thermodynamic systems, or physical systems that are not thermodynamic systems. A wall of a thermodynamic system may be purely notional, when it is described as being 'permeable' to all matter, all radiation, and all forces.

Thermodynamics is expressed by a mathematical framework of thermodynamic equations which relate various thermodynamic quantities and physical properties measured in a laboratory or production process. Thermodynamics is based on a fundamental set of postulates, that became the laws of thermodynamics.
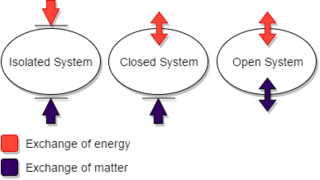
In physical science, an isolated system is either of the following:
- a physical system so far removed from other systems that it does not interact with them.
- a thermodynamic system enclosed by rigid immovable walls through which neither mass nor energy can pass.

In thermodynamics, the internal energy of a system is expressed in terms of pairs of conjugate variables such as temperature and entropy or pressure and volume. In fact, all thermodynamic potentials are expressed in terms of conjugate pairs. The product of two quantities that are conjugate has units of energy or sometimes power.

Classical thermodynamics considers three main kinds of thermodynamic process by change in a system, cycles in a system, and flow processes.

In thermodynamics, work performed by a system is energy transferred by the system to its surroundings, by a mechanism through which the system can spontaneously exert macroscopic forces on its surroundings. In the surroundings, through suitable passive linkages, the work can lift a weight, for example. Energy can also transfer from the surroundings to the system; in a sign convention used in physics, such work has a negative magnitude.
In classical thermodynamics, entropy is a property of a thermodynamic system that expresses the direction or outcome of spontaneous changes in the system. The term was introduced by Rudolf Clausius in the mid-nineteenth century from the Greek word τρoπή (transformation) to explain the relationship of the internal energy that is available or unavailable for transformations in form of heat and work. Entropy predicts that certain processes are irreversible or impossible, despite not violating the conservation of energy. The definition of entropy is central to the establishment of the second law of thermodynamics, which states that the entropy of isolated systems cannot decrease with time, as they always tend to arrive at a state of thermodynamic equilibrium, where the entropy is highest. Entropy is therefore also considered to be a measure of disorder in the system.

In the history of thermodynamics, On the Equilibrium of Heterogeneous Substances is a 300-page paper written by American chemical physicist Willard Gibbs. It is one of the founding papers in thermodynamics, along with German physicist Hermann von Helmholtz's 1882 paper "Thermodynamik chemischer Vorgänge." Together they form the foundation of chemical thermodynamics as well as a large part of physical chemistry.

In thermodynamics, heat is energy in transfer to or from a thermodynamic system, by mechanisms other than thermodynamic work or transfer of matter. The various mechanisms of energy transfer that define heat are stated in the next section of this article.
In thermodynamics, a thermally isolated system can exchange no mass or heat energy with its environment. The internal energy of a thermally isolated system may therefore change due to the exchange of work energy. The entropy of a thermally isolated system will increase in time if it is not at equilibrium, but as long as it is at equilibrium, its entropy will be at a maximum and constant value and will not change, no matter how much work energy the system exchanges with its environment. To maintain this constant entropy, any exchange of work energy with the environment must therefore be quasistatic in nature, in order to assure that the system remains essentially at equilibrium during the process.





