
Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure or mixing with a catalyst. Heat is not necessarily applied externally, and is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.

Phenol formaldehyde resins (PF) are synthetic polymers obtained by the reaction of phenol or substituted phenol with formaldehyde. Used as the basis for Bakelite, PFs were the first commercial synthetic resins (plastics). They have been widely used for the production of molded products including billiard balls, laboratory countertops, and as coatings and adhesives. They were at one time the primary material used for the production of circuit boards but have been largely replaced with epoxy resins and fiberglass cloth, as with fire-resistant FR-4 circuit board materials.

Formica Laminate is a laminated composite material invented at the Westinghouse Electric Corporation in the United States in 1912. Originally used to replace mica in electrical applications, it has since been manufactured for multiple applications. It has been produced by Formica Group manufacturing sites across the globe since. Formica Group are best known for the company's classic product: a heat-resistant, wipe-clean laminate of paper with melamine resin.

Lamination is the technique/process of manufacturing a material in multiple layers, so that the composite material achieves improved strength, stability, sound insulation, appearance, or other properties from the use of the differing materials, such as plastic. A laminate is a permanently assembled object created using heat, pressure, welding, or adhesives. Various coating machines, machine presses and calendering equipment are used.
FR-4 is a NEMA grade designation for glass-reinforced epoxy laminate material. FR-4 is a composite material composed of woven fiberglass cloth with an epoxy resin binder that is flame resistant (self-extinguishing).

Electrophoretic deposition (EPD), is a term for a broad range of industrial processes which includes electrocoating, cathodic electrodeposition, anodic electrodeposition, and electrophoretic coating, or electrophoretic painting. A characteristic feature of this process is that colloidal particles suspended in a liquid medium migrate under the influence of an electric field (electrophoresis) and are deposited onto an electrode. All colloidal particles that can be used to form stable suspensions and that can carry a charge can be used in electrophoretic deposition. This includes materials such as polymers, pigments, dyes, ceramics and metals.
FR-2 is a NEMA designation for synthetic resin bonded paper, a composite material made of paper impregnated with a plasticized phenol formaldehyde resin, used in the manufacture of printed circuit boards. Its main properties are similar to NEMA grade XXXP (MIL-P-3115) material, and can be substituted for the latter in many applications.
Micarta is a brand name for composites of linen, canvas, paper, fiberglass, carbon fiber, or other fabric in a thermosetting plastic. It was originally used in electrical and decorative applications. Micarta was developed by George Westinghouse at least as early as 1910 using phenolic resins invented by Leo Baekeland. These resins were used to impregnate paper and cotton fabric which were cured under pressure and high temperature to produce laminates. In later years this manufacturing method included the use of fiberglass fabric, and other resin types were also used. Today Micarta high-pressure industrial laminates are produced with a wide variety of resins and fibers. The term has been used generically for most resin impregnated fiber compounds. Common uses of modern high-pressure laminates include electrical insulators, printed circuit board substrates, and knife handles.
Wood glue is an adhesive used to tightly bond pieces of wood together. Many substances have been used as glues. Traditionally animal proteins like casein from milk or collagen from animal hides and bones were boiled down to make early glues. They worked by solidifying as they dried. Later, glues were made from plant starches like flour or potato starch. When combined with water and heated, the starch gelatinizes and forms a sticky paste as it dries. Plant-based glues were common for books and paper products, though they can break down more easily over time compared to animal-based glues. Examples of modern wood glues include polyvinyl acetate (PVA) and epoxy resins. Some resins used in producing composite wood products may contain formaldehyde. As of 2021, “the wood panel industry uses almost 95% of synthetic petroleum-derived thermosetting adhesives, mainly based on urea, phenol, and melamine, among others”.

An intumescent is a substance that swells as a result of heat exposure, leading to an increase in volume and decrease in density. Intumescence refers to the process of swelling. Intumescent materials are typically used in passive fire protection and require listing, approval, and compliance in their installed configurations in order to comply with the national building codes and laws.

Self-healing materials are artificial or synthetically created substances that have the built-in ability to automatically repair damages to themselves without any external diagnosis of the problem or human intervention. Generally, materials will degrade over time due to fatigue, environmental conditions, or damage incurred during operation. Cracks and other types of damage on a microscopic level have been shown to change thermal, electrical, and acoustical properties of materials, and the propagation of cracks can lead to eventual failure of the material. In general, cracks are hard to detect at an early stage, and manual intervention is required for periodic inspections and repairs. In contrast, self-healing materials counter degradation through the initiation of a repair mechanism that responds to the micro-damage. Some self-healing materials are classed as smart structures, and can adapt to various environmental conditions according to their sensing and actuation properties.
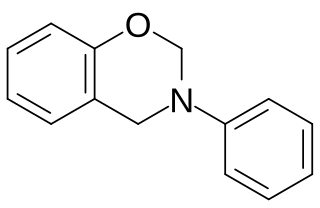
Polybenzoxazines, also called benzoxazine resins, are cured polymerization products derived from benzoxazine monomers.
Polyester resins are synthetic resins formed by the reaction of dibasic organic acids and polyhydric alcohols. Maleic anhydride is a commonly used raw material with diacid functionality in unsaturated polyester resins. Unsaturated polyester resins are used in sheet moulding compound, bulk moulding compound and the toner of laser printers. Wall panels fabricated from polyester resins reinforced with fiberglass—so-called fiberglass reinforced plastic (FRP)—are typically used in restaurants, kitchens, restrooms and other areas that require washable low-maintenance walls. They are also used extensively in cured-in-place pipe applications. Departments of Transportation in the USA also specify them for use as overlays on roads and bridges. In this application they are known AS Polyester Concrete Overlays (PCO). These are usually based on isophthalic acid and cut with styrene at high levels—usually up to 50%. Polyesters are also used in anchor bolt adhesives though epoxy based materials are also used. Many companies have and continue to introduce styrene free systems mainly due to odor issues, but also over concerns that styrene is a potential carcinogen. Drinking water applications also prefer styrene free. Most polyester resins are viscous, pale coloured liquids consisting of a solution of a polyester in a reactive diluent which is usually styrene, but can also include vinyl toluene and various acrylates.
Polymer engineering is generally an engineering field that designs, analyses, and modifies polymer materials. Polymer engineering covers aspects of the petrochemical industry, polymerization, structure and characterization of polymers, properties of polymers, compounding and processing of polymers and description of major polymers, structure property relations and applications.
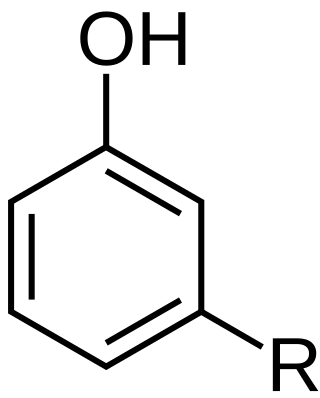
Cardanol is a phenolic lipid obtained from anacardic acid, the main component of cashew nutshell liquid (CNSL), a byproduct of cashew nut processing. Cardanol finds use in the chemical industry in resins, coatings, frictional materials, and surfactants used as pigment dispersants for water-based inks. It is used to make phenalkamines, which are used as curing agents for the durable epoxy coatings used on concrete floors. The name of the substance is derived by contraction from the genus Anacardium, which includes the cashew tree, Anacardium occidentale. The name of the genus itself is based on the Greek word for heart.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle.
The wet strength of paper and paperboard is a measure of how well the web of fibers holding the paper together can resist a force of rupture when the paper is wet. Wet strength is routinely expressed as the ratio of wet to dry tensile force at break.

Decorative laminates are laminated products primarily used as furniture surface materials or wall paneling. It can be manufactured as either high- or low-pressure laminate, with the two processes not much different from each other except for the pressure applied in the pressing process. Also, laminate can be produced either in batches or in a continuous process; the latter is called continuous pressure laminate (CPL).
Impregnation resins are slightly viscous, organic liquids that are used in the forest products industry for wood modification. They typically contain formaldehyde and are composed of dimers and trimers of the main molecule. These can become polymer solutions upon curing inside of a wood substrate, imparting stabilizing properties. Impregnation of these resins involves a vacuum chamber procedure that completely disperses the resin into the wood. Once inside of the wood, the resin can diffuse into the cell wall and enhance the physical strength of the wood even further.














