Related Research Articles

Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While activation energy must be supplied to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.

A flame is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density they are then considered plasma.
A combustor is a component or area of a gas turbine, ramjet, or scramjet engine where combustion takes place. It is also known as a burner, burner can, combustion chamber or flame holder. In a gas turbine engine, the combustor or combustion chamber is fed high-pressure air by the compression system. The combustor then heats this air at constant pressure as the fuel/air mix burns. As it burns the fuel/air mix heats and rapidly expands. The burned mix is exhausted from the combustor through the nozzle guide vanes to the turbine. In the case of a ramjet or scramjet engines, the exhaust is directly fed out through the nozzle.
Homogeneous Charge Compression Ignition (HCCI) is a form of internal combustion in which well-mixed fuel and oxidizer are compressed to the point of auto-ignition. As in other forms of combustion, this exothermic reaction produces heat that can be transformed into work in a heat engine.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.

A premixed flame is a flame formed under certain conditions during the combustion of a premixed charge of fuel and oxidiser. Since the fuel and oxidiser—the key chemical reactants of combustion—are available throughout a homogeneous stoichiometric premixed charge, the combustion process once initiated sustains itself by way of its own heat release. The majority of the chemical transformation in such a combustion process occurs primarily in a thin interfacial region which separates the unburned and the burned gases. The premixed flame interface propagates through the mixture until the entire charge is depleted. The propagation speed of a premixed flame is known as the flame speed which depends on the convection-diffusion-reaction balance within the flame, i.e. on its inner chemical structure. The premixed flame is characterised as laminar or turbulent depending on the velocity distribution in the unburned pre-mixture.

A thermal oxidizer is a process unit for air pollution control in many chemical plants that decomposes hazardous gases at a high temperature and releases them into the atmosphere.

HCNG or H2CNG is a mixture of compressed natural gas and 4–9 percent hydrogen by energy. It may be used as a fuel gas for internal combustion engines and home appliances.
Flame lift-off in oil fired pressure jet burners is an unwanted condition in which the flame and burner become separated. This condition is most commonly created by excessive combustion air and often results in the loss of flame as the photoelectric cell fails to register the light of the flame, this in turn results in a safety lockout of the control box.
In combustion engineering and explosion studies, the Markstein number characterizes the effect of local heat release of a propagating flame on variations in the surface topology along the flame and the associated local flame front curvature. The dimensionless Markstein number is defined as:
A cool flame or invisible flame is a flame having a typical temperature of about 400 °C (752 °F). It is usually produced in a chemical reaction of a certain fuel-air mixture. In contrast to an ordinary flame, the reaction is not vigorous and releases little heat, light, or carbon dioxide. Cold flames are difficult to observe and are uncommon in everyday life, but they are responsible for engine knock – the undesirable, erratic, and noisy combustion of low-octane fuels in internal combustion engines.
A shock-induced combustion ramjet engine (abbreviated as shcramjet; also called oblique detonation wave engine; also called standing oblique detonation ramjet (sodramjet); or simply referred to as shock-ramjet engine) is a concept of air-breathing ramjet engine, proposed to be used for hypersonic and/or single-stage-to-orbit propulsion applications.
Combustion models for CFD refers to combustion models for computational fluid dynamics. Combustion is defined as a chemical reaction in which a hydrocarbon fuel reacts with an oxidant to form products, accompanied with the release of energy in the form of heat. Being the integral part of various engineering applications like: internal combustion engines, aircraft engines, rocket engines, furnaces, and power station combustors, combustion manifests itself as a wide domain during the design, analysis and performance characteristics stages of the above-mentioned applications. With the added complexity of chemical kinetics and achieving reacting flow mixture environment, proper modeling physics has to be incorporated during computational fluid dynamic (CFD) simulations of combustion. Hence the following discussion presents a general outline of the various adequate models incorporated with the Computational fluid dynamic code to model the process of combustion.
The SRM Engine Suite is an engineering software tool used for simulating fuels, combustion and exhaust gas emissions in internal combustion engine applications. It is used worldwide by leading IC engine development organisations and fuel companies. The software is developed, maintained and supported by CMCL Innovations, Cambridge, U.K.
Heterogeneous combustion, otherwise known as combustion in porous media, is a type of combustion in which a solid and gas phase interact to promote the complete transfer of reactants to their lower energy potential products. In this type of combustion a high surface area solid is immersed into a gaseous reacting flow, additional fluid phases may or may not be present. Chemical reactions and heat transfer occur locally on each phase and between both phases. Heterogeneous Combustion differs from catalysis as there is no focus to either phase individually but rather both examined simultaneously. In some materials, such as silicon carbide (SiC), oxide layers, SiO and SiO2, which form on the surface enable the adsorption of water vapor from the gas phase onto the solid lowering partial pressures. In this regime of combustion, thermal heat released from the combustion byproducts are transferred into the solid phase by convection; conduction and radiation both then conduct heat upstream (along with adverse convection within the gas phase). Heat is then convectively transferred to the unburnt reactants.

A rotating detonation engine (RDE) is an engine using a form of pressure gain combustion, where one or more detonations continuously travel around an annular channel. Computational simulations and experimental results have shown that the RDE has potential in transport and other applications.
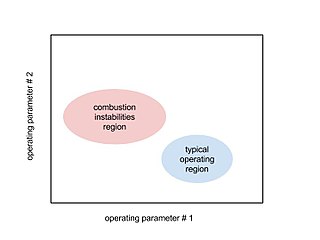
Combustion instabilities are physical phenomena occurring in a reacting flow in which some perturbations, even very small ones, grow and then become large enough to alter the features of the flow in some particular way.

Ashwani K. Gupta is a British-American engineer and educator with research focus on combustion, fuels, fuel reforming, advanced diagnostics, High Temperature Air Combustion, and high-intensity distributed combustion, green combustion turbine, micro-combustion, and air pollution. He is an Distinguished University Professor at the University of Maryland. Gupta is also Professor of Mechanical Engineering at the University of Maryland and Director of Combustion Laboratory. He is also an Affiliate Professor at Institute of Physical Science and Technology, University of Maryland which is part of the University of Maryland College of Computer, Mathematical and Natural Sciences.
David S-K Ting is a Canadian academic, author and researcher. He is a professor of mechanical, automotive & materials engineering at the University of Windsor. He is the founder of the Turbulence & Energy Laboratory.
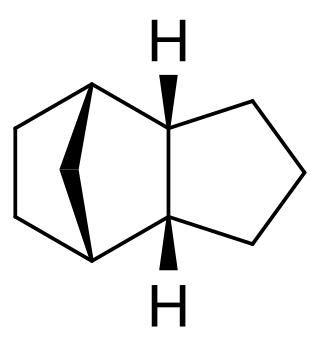
Tricyclodecane (TCD) is an organic compound with the formula C10H16. It is classed as a hydrocarbon. It has two main stereoisomers–the endo and exo forms. Its primary use in the exo form is as a component of jet fuel. It is used here primarily because of its high energy density. The exo isomer also has a low freezing point. Because of this, its properties have been studied extensively. It is often called tetrahydrodicyclopentadiene.
References
- ↑ Kuo, C.H.; Ronney, P.D. (January 2007). "Numerical modeling of on-adiabatic heat-recirculating combustors". Proceedings of the Combustion Institute. 32 (2): 3277–3284. doi:10.1016/j.proci.2006.08.082.
- ↑ Kim, Nam Il; Kato, Souichiro; Kataoka, Takuya; Yokomori, Takeshi; Maruyama, Shigenao; Fujimori, Toshiro; Maruta, Kaoru (May 2005). "Flame stabilization and emission of small swiss-roll combustors as heaters". Combustion and Flame . 141 (3): 229–240. doi:10.1016/j.combustflame.2005.01.006.
- ↑ Epstein, A.H.; Senturia, S.D. (May 23, 1997). "Macro power from micro machinery". Science . 276 (5316): 1211. doi:10.1126/science.276.5316.1211. S2CID 110839795.
- ↑ Fernandez-Pello, A. Carlos (2002). "Micro-power generation using combustion: issues and approaches" (PDF). Proceedings of the Combustion Institute. 29 (1): 883–899. doi:10.1016/S1540-7489(02)80113-4.
- ↑ Pattekar, A.V.; Kothare, M.V. (February 2004). "A microreactor for hydrogen production in micro fuel cell applications". Journal of Microelectromechanical Systems. 13 (1): 7–18. doi:10.1109/JMEMS.2004.823224. S2CID 19243473.
- ↑ Khandelwal, Bhupendra; Sahota, Gur Partap Singh; Kumar, Sudarshan (August 27, 2010). "Investigations into the flame stability limits in a backward step micro scale combustor with premixed methane–air mixtures". Journal of Micromechanics and Microengineering . 20 (9): 095030. doi:10.1088/0960-1317/20/9/095030.
- ↑ Maruta, K.; Kataoka, T.; Kim, N.I.; Minaev, S.; Fursenko, R. (January 2005). "Characteristics of combustion in a narrow channel with a temperature gradient". Proceedings of the Combustion Institute. 30 (2): 2429–2436. doi:10.1016/j.proci.2004.08.245.
- ↑ Weinberg, Felix (September 2004). "Optimizing heat recirculating combustion systems for thermoelectric converters". Combustion and Flame . 138 (4): 401–403. doi:10.1016/j.combustflame.2004.06.007.
- ↑ Shih, Hsin-Yi; Huang, Yen-Chin (June 2009). "Thermal design and model analysis of the swiss-roll recuperator for an innovative micro gas turbine". Applied Thermal Engineering . 29 (8–9): 1493–1499. doi:10.1016/j.applthermaleng.2008.06.029.
- ↑ Yang, W.M.; Chou, S.K.; Shu, C.; Xue, H.; Lil, Z.W. (March 17, 2004). "Development of a prototype micro-thermophotovoltaic power generator". Journal of Physics D: Applied Physics. 37 (7): 1017–1020. doi:10.1088/0022-3727/37/7/011.
- ↑ Aichlmayr, H.T.; Kittelson, D.B.; Zachariah, M.R. (November 2003). "Micro-HCCI combustion: experimental characterization and development of a detailed chemical kinetic model with coupled piston motion". Combustion and Flame . 135 (3): 227–248. doi:10.1016/S0010-2180(03)00161-5.
- ↑ Li, Junwei; Zhong, Beijing (May 2008). "Experimental investigation on heat loss and combustion in methane/oxygen micro-tube combustor". Applied Thermal Engineering . 28 (7): 707–716. doi:10.1016/j.applthermaleng.2007.06.001.
- ↑ Kumar, Sudarshan; Maruta, Kaoru; Minaev, S. (April 3, 2007). "Experimental investigations on the combustion behavior of methane-air mixtures in a new micro scale radial combustor configuration". Journal of Micromechanics and Microengineering . 17 (5): 900–908. doi:10.1088/0960-1317/17/5/008.
- ↑ Kumar, S.; Minaev, S.; Maruta, S.K. (January 2007). "On the formation of multiple rotating pelton-like flame structures in radial microchannels with lean methane-air mixtures". Proceedings of the Combustion Institute. 31 (2): 3261–3268. doi:10.1016/j.proci.2006.07.174.
- ↑ Khandelwal, Bhupendra; Kumar, Sudarshan (December 2010). "Experimental investigations on flame stabilization behavior in a diverging micro channel with premixed methane-air mixtures". Applied Thermal Engineering . 30 (17–18): 2718–2723. doi:10.1016/j.applthermaleng.2010.07.023.