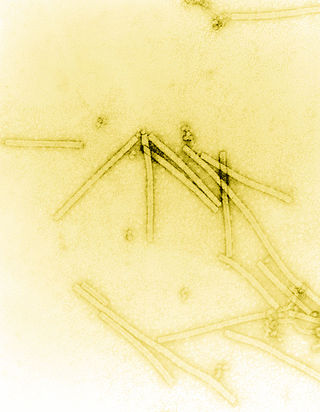
Tobacco mosaic virus (TMV) is a positive-sense single-stranded RNA virus species in the genus Tobamovirus that infects a wide range of plants, especially tobacco and other members of the family Solanaceae. The infection causes characteristic patterns, such as "mosaic"-like mottling and discoloration on the leaves. TMV was the first virus to be discovered. Although it was known from the late 19th century that a non-bacterial infectious disease was damaging tobacco crops, it was not until 1930 that the infectious agent was determined to be a virus. It is the first pathogen identified as a virus. The virus was crystallised by Wendell Meredith Stanley. It has a similar size to the largest synthetic molecule, known as PG5.

Rhabdoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates, invertebrates, plants, fungi and protozoans serve as natural hosts. Diseases associated with member viruses include rabies encephalitis caused by the rabies virus, and flu-like symptoms in humans caused by vesiculoviruses. The name is derived from Ancient Greek rhabdos, meaning rod, referring to the shape of the viral particles. The family has 40 genera, most assigned to three subfamilies.

Sedoreoviridae is a family of double-stranded RNA viruses. Member viruses have a wide host range, including vertebrates, invertebrates, plants, protists and fungi. They lack lipid envelopes and package their segmented genome within multi-layered capsids. Lack of a lipid envelope has allowed three-dimensional structures of these large complex viruses to be obtained, revealing a structural and likely evolutionary relationship to the cystovirus family of bacteriophage. There are currently 97 species in this family, divided among 15 genera in two subfamilies. Reoviruses can affect the gastrointestinal system and respiratory tract. The name "reo-" is an acronym for "respiratory enteric orphan" viruses. The term "orphan virus" refers to the fact that some of these viruses have been observed not associated with any known disease. Even though viruses in the family Reoviridae have more recently been identified with various diseases, the original name is still used.

Plant viruses are viruses that affect plants. Like all other viruses, plant viruses are obligate intracellular parasites that do not have the molecular machinery to replicate without a host. Plant viruses can be pathogenic to vascular plants.

Geminiviridae is a family of plant viruses that encode their genetic information on a circular genome of single-stranded (ss) DNA. There are 520 species in this family, assigned to 14 genera. Diseases associated with this family include: bright yellow mosaic, yellow mosaic, yellow mottle, leaf curling, stunting, streaks, reduced yields. They have single-stranded circular DNA genomes encoding genes that diverge in both directions from a virion strand origin of replication. According to the Baltimore classification they are considered class II viruses. It is the largest known family of single stranded DNA viruses.

Tombusviridae is a family of single-stranded positive sense RNA plant viruses. There are three subfamilies, 17 genera, and 95 species in this family. The name is derived from Tomato bushy stunt virus (TBSV).

Plasmodesmata are microscopic channels which traverse the cell walls of plant cells and some algal cells, enabling transport and communication between them. Plasmodesmata evolved independently in several lineages, and species that have these structures include members of the Charophyceae, Charales, Coleochaetales and Phaeophyceae, as well as all embryophytes, better known as land plants. Unlike animal cells, almost every plant cell is surrounded by a polysaccharide cell wall. Neighbouring plant cells are therefore separated by a pair of cell walls and the intervening middle lamella, forming an extracellular domain known as the apoplast. Although cell walls are permeable to small soluble proteins and other solutes, plasmodesmata enable direct, regulated, symplastic transport of substances between cells. There are two forms of plasmodesmata: primary plasmodesmata, which are formed during cell division, and secondary plasmodesmata, which can form between mature cells.

Tobamovirus is a genus of positive-strand RNA viruses in the family Virgaviridae. Many plants, including tobacco, potato, tomato, and squash, serve as natural hosts. Diseases associated with this genus include: necrotic lesions on leaves. The name Tobamovirus comes from the host and symptoms of the first virus discovered.

Tomato bushy stunt virus (TBSV) is a virus of the tombusvirus family. It was first reported in tomatoes in 1935 and primarily affects vegetable crops, though it is not generally considered an economically significant plant pathogen. Depending upon the host, TBSV causes stunting of growth, leaf mottling, and deformed or absent fruit. The virus is likely to be soil-borne in the natural setting, but can also be transmitted mechanically, for example through contaminated cutting tools. TBSV has been used as a model system in virology research on the life cycle of plant viruses, particularly in experimental infections of the model host plant Nicotiana benthamiana.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.

Potyvirus is a genus of positive-strand RNA viruses in the family Potyviridae. Plants serve as natural hosts. Like begomoviruses, members of this genus may cause significant losses in agricultural, pastoral, horticultural, and ornamental crops. More than 200 species of aphids spread potyviruses, and most are from the subfamily Aphidinae. The genus contains 190 species and potyviruses account for about thirty percent of all currently known plant viruses.
Phycodnaviridae is a family of large (100–560 kb) double-stranded DNA viruses that infect marine or freshwater eukaryotic algae. Viruses within this family have a similar morphology, with an icosahedral capsid. As of 2014, there were 33 species in this family, divided among 6 genera. This family belongs to a super-group of large viruses known as nucleocytoplasmic large DNA viruses. Evidence was published in 2014 suggesting that specific strains of Phycodnaviridae might infect humans rather than just algal species, as was previously believed. Most genera under this family enter the host cell by cell receptor endocytosis and replicate in the nucleus. Phycodnaviridae play important ecological roles by regulating the growth and productivity of their algal hosts. Algal species such Heterosigma akashiwo and the genus Chrysochromulina can form dense blooms which can be damaging to fisheries, resulting in losses in the aquaculture industry. Heterosigma akashiwo virus (HaV) has been suggested for use as a microbial agent to prevent the recurrence of toxic red tides produced by this algal species. Phycodnaviridae cause death and lysis of freshwater and marine algal species, liberating organic carbon, nitrogen and phosphorus into the water, providing nutrients for the microbial loop.
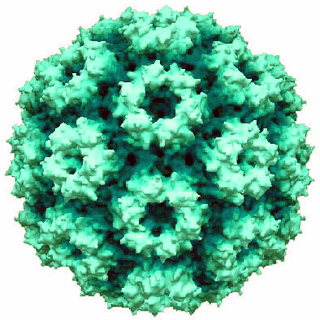
Cowpea chlorotic mottle virus, known by the abbreviation CCMV, is a virus that specifically infects the cowpea plant, or black-eyed pea. The leaves of infected plants develop yellow spots, hence the name "chlorotic". Similar to its "brother" virus, Cowpea mosaic virus (CPMV), CCMV is produced in high yield in plants. In the natural host, viral particles can be produced at 1–2 mg per gram of infected leaf tissue. Belonging to the bromovirus genus, cowpea chlorotic mottle virus (CCMV) is a small spherical plant virus. Other members of this genus include the brome mosaic virus (BMV) and the broad bean mottle virus (BBMV).

Cucumber mosaic virus (CMV) is a plant pathogenic virus in the family Bromoviridae. This virus has a worldwide distribution and a very wide host range, having the reputation of the widest host range of any known plant virus. It can be transmitted from plant to plant both mechanically by sap and by aphids in a stylet-borne fashion. It can also be transmitted in seeds and by the parasitic weeds, Cuscuta sp. (dodder).
Idaeovirus is a genus of positive-sense ssRNA viruses that contains two species: Raspberry bushy dwarf virus (RBDV) and Privet idaeovirus. RBDV has two host-dependent clades: one for raspberries; the other for grapevines. Infections are a significant agricultural burden, resulting in decreased yield and quality of crops. RBDV has a synergistic relation with Raspberry leaf mottle virus, with co-infection greatly amplifying the concentration of virions in infected plants. The virus is transmitted via pollination with RBDV-infected pollen grains that first infect the stigma before causing systemic infection.

Nonstructural protein 5A (NS5A) is a zinc-binding and proline-rich hydrophilic phosphoprotein that plays a key role in Hepatitis C virus RNA replication. It appears to be a dimeric form without trans-membrane helices.

Fig mosaic emaravirus (FMV) is a segmented, negative sense, single-stranded RNA virus that is determined to be the causal agent of fig mosaic disease (FMD) in fig plants, Ficus carica. It is a member of the genus Emaravirus and order Bunyavirales and is transmitted mainly by the eriophyid mite Aceria ficus. FMV can cause a range of symptoms varying in severity, including leaf chlorosis, deformity, and mosaic or discoloration patterns, as well as premature fruit drop.
Lily virus X (LVX) is a pathogenic ssRNA(+) plant virus of the family Alphaflexiviridae and the order Tymovirales.

The Blueberry mosaic associated ophiovirus (B1MaV) is a plant virus which infects blueberry plants, causing a discoloration of the leaves of the plants in a mosaic-like pattern. The disease is found in blueberry plants in many regions of North America, as well as South America, Europe, New Zealand, and South Africa. Within these regions the virus is most often found in high blueberry-yielding areas, but can be spread to other locations. Blueberry mosaic associatedophiovirus is one of seven species in the genus Ophiovirus. It is a member of the Aspiviridae family, in the Serpentovirales order, and in the Milnevircetes class. The Ophioviridae viruses are characterized by a flexible and elongated nucleocapsid that is composed mostly of filamentous structures and is helically symmetrical. It also has a non-enveloped protein capsid that is capable of coiling around itself allowing for a super-coiled structure and the helical symmetry. The virus has the potential to be symptomatic or asymptomatic within plants causing the display of symptoms in only a few plants, but the ability to transmit the virus unknowingly in many plants. B1MaV often remains asymptomatic for long periods of time after initial infection allowing for blind transmission.
















