
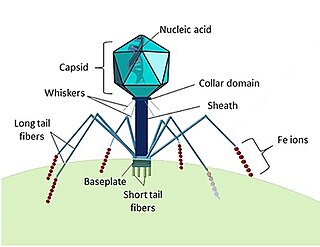
A bacteriophage, also known informally as a phage, is a duplodnaviria virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν, meaning "to devour". Bacteriophages are composed of proteins that encapsulate a DNA or RNA genome, and may have structures that are either simple or elaborate. Their genomes may encode as few as four genes and as many as hundreds of genes. Phages replicate within the bacterium following the injection of their genome into its cytoplasm.

A mangrove is a shrub or tree that grows mainly in coastal saline or brackish water. Mangroves grow in an equatorial climate, typically along coastlines and tidal rivers. They have special adaptations to take in extra oxygen and to remove salt, which allow them to tolerate conditions that would kill most plants. The term is also used for tropical coastal vegetation consisting of such species. Mangroves are taxonomically diverse, as a result of convergent evolution in several plant families. They occur worldwide in the tropics and subtropics and even some temperate coastal areas, mainly between latitudes 30° N and 30° S, with the greatest mangrove area within 5° of the equator. Mangrove plant families first appeared during the Late Cretaceous to Paleocene epochs, and became widely distributed in part due to the movement of tectonic plates. The oldest known fossils of mangrove palm date to 75 million years ago.

Metagenomics is the study of genetic material recovered directly from environmental or clinical samples by a method called sequencing. The broad field may also be referred to as environmental genomics, ecogenomics, community genomics or microbiomics.
Marnaviridae is a family of positive-stranded RNA viruses in the order Picornavirales that infect various photosynthetic marine protists. Members of the family have non-enveloped, icosahedral capsids. Replication occurs in the cytoplasm and causes lysis of the host cell. The first species of this family that was isolated is Heterosigma akashiwo RNA virus (HaRNAV) in the genus Marnavirus, which infects the toxic bloom-forming Raphidophyte alga, Heterosigma akashiwo. As of 2021, there are twenty species across seven genera in this family, as well as many other related virus sequences discovered through metagenomic sequencing that are currently unclassified.
MEGAN is a computer program that allows optimized analysis of large metagenomic datasets.

Virophages are small, double-stranded DNA viral phages that require the co-infection of another virus. The co-infecting viruses are typically giant viruses. Virophages rely on the viral replication factory of the co-infecting giant virus for their own replication. One of the characteristics of virophages is that they have a parasitic relationship with the co-infecting virus. Their dependence upon the giant virus for replication often results in the deactivation of the giant viruses. The virophage may improve the recovery and survival of the host organism.

A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea. Since Dmitri Ivanovsky's 1892 article describing a non-bacterial pathogen infecting tobacco plants and the discovery of the tobacco mosaic virus by Martinus Beijerinck in 1898, more than 11,000 of the millions of virus species have been described in detail. Viruses are found in almost every ecosystem on Earth and are the most numerous type of biological entity. The study of viruses is known as virology, a subspeciality of microbiology.

Forest Rohwer is an American microbial ecologist and Professor of Biology at San Diego State University. His particular interests include coral reef microbial ecology and viruses as both evolutionary agents and opportunistic pathogens in various environments.

Alexandra (Alex) Z. Worden is a microbial ecologist and genome scientist known for her expertise in the ecology and evolution of ocean microbes and their influence on global biogeochemical cycles.

The human virome is the total collection of viruses in and on the human body. Viruses in the human body may infect both human cells and other microbes such as bacteria. Some viruses cause disease, while others may be asymptomatic. Certain viruses are also integrated into the human genome as proviruses or endogenous viral elements.

Viral metagenomics is the metagenomic study of viral genetic material obtained from environmental DNA samples or clinical DNA samples obtained from a host or natural reservoir. Metagenomic methods can be applied to study viruses in any system and has been used to describe various viruses associated with cancerous tumors, extreme environments, terrestrial ecosystems, and the blood and feces of humans. The term virome is also used to refer to viruses investigated by metagenomic sequencing of viral nucleic acids and is frequently used to describe environmental shotgun metagenomes. Viral metagenomics is a culture independent methodology that provides insights on viral diversity, abundance, and functional potential of viruses within the environment. Viruses lack a universal phylogenetic marker making metagenomics the only way to assess the genetic diversity of viruses in an environmental sample. With the advancements of techniques that can exploit next-generation sequencing, viruses can now be studied outside of culturable virus-host pairs. This approach has created improvements in molecular epidemiology and accelerated the discovery of novel viruses.

Virome refers to the assemblage of viruses that is often investigated and described by metagenomic sequencing of viral nucleic acids that are found associated with a particular ecosystem, organism or holobiont. The word is frequently used to describe environmental viral shotgun metagenomes. Viruses, including bacteriophages, are found in all environments, and studies of the virome have provided insights into nutrient cycling, development of immunity, and a major source of genes through lysogenic conversion. Also, the human virome has been characterized in nine organs of 31 Finnish individuals using qPCR and NGS methodologies.

Genomoviridae is a family of single stranded DNA viruses that mainly infect fungi. The genomes of this family are small. The genomes are circular single-stranded DNA and encode rolling-circle replication initiation proteins (Rep) and unique capsid proteins. In Rep-based phylogenies, genomoviruses form a sister clade to plant viruses of the family Geminiviridae. Ten genera are recognized in this family.
Auxiliary metabolic genes (AMGs) are found in many bacteriophages but originated in bacterial cells. AMGs modulate host cell metabolism during infection so that the phage can replicate more efficiently. For instance, bacteriophages that infect the abundant marine cyanobacteria Synechococcus and Prochlorococcus (cyanophages) carry AMGs that have been acquired from their immediate host as well as more distantly-related bacteria. Cyanophage AMGs support a variety of functions including photosynthesis, carbon metabolism, nucleic acid synthesis and metabolism.

Redondoviruses are a family of human-associated DNA viruses. Their name derives from the inferred circular structure of the viral genome . Redondoviruses have been identified in DNA sequence based surveys of samples from humans, primarily samples from the oral cavity and upper airway.
Marine viruses are defined by their habitat as viruses that are found in marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. Viruses are small infectious agents that can only replicate inside the living cells of a host organism, because they need the replication machinery of the host to do so. They can infect all types of life forms, from animals and plants to microorganisms, including bacteria and archaea.

Varidnaviria is a realm of viruses that includes all DNA viruses that encode major capsid proteins that contain a vertical jelly roll fold. The major capsid proteins (MCP) form into pseudohexameric subunits of the viral capsid, which stores the viral deoxyribonucleic acid (DNA), and are perpendicular, or vertical, to the surface of the capsid. Apart from this, viruses in the realm also share many other characteristics, such as minor capsid proteins (mCP) with the vertical jelly roll fold, an ATPase that packages viral DNA into the capsid, and a DNA polymerase that replicates the viral genome.
Nucleocytoviricota is a phylum of viruses. Members of the phylum are also known as the nucleocytoplasmic large DNA viruses (NCLDV), which serves as the basis of the name of the phylum with the suffix -viricota for virus phylum. These viruses are referred to as nucleocytoplasmic because they are often able to replicate in both the host's cell nucleus and cytoplasm.
Virosphere was coined to refer to all those places in which viruses are found or which are affected by viruses. However, more recently virosphere has also been used to refer to the pool of viruses that occurs in all hosts and all environments, as well as viruses associated with specific types of hosts, type of genome or ecological niche.
Ian Hewson is an Australian American biological oceanographer and marine ecologist who is a professor of microbiology at Cornell University. He leads the Cornell Marine Mass Mortality Laboratory, where he studies the drives of marine mass mortalities. He is leader of diversity, equity, and inclusion for the Department of Microbiology.















