Related Research Articles

Senescence or biological aging is the gradual deterioration of functional characteristics in living organisms. The word senescence can refer to either cellular senescence or to senescence of the whole organism. Organismal senescence involves an increase in death rates and/or a decrease in fecundity with increasing age, at least in the later part of an organism's life cycle. but it can be delayed. The 1934 discovery that calorie restriction can extend lifespans by 50% in rats, the existence of species having negligible senescence, and the existence of potentially immortal organisms such as members of the genus Hydra have motivated research into delaying senescence and thus age-related diseases. Rare human mutations can cause accelerated aging diseases.
Maximum life span is a measure of the maximum amount of time one or more members of a population have been observed to survive between birth and death. The term can also denote an estimate of the maximum amount of time that a member of a given species could survive between birth and death, provided circumstances that are optimal to that member's longevity.
The free radical theory of aging states that organisms age because cells accumulate free radical damage over time. A free radical is any atom or molecule that has a single unpaired electron in an outer shell. While a few free radicals such as melanin are not chemically reactive, most biologically relevant free radicals are highly reactive. For most biological structures, free radical damage is closely associated with oxidative damage. Antioxidants are reducing agents, and limit oxidative damage to biological structures by passivating them from free radicals.

Biogerontology is the sub-field of gerontology concerned with the biological aging process, its evolutionary origins, and potential means to intervene in the process. The term "biogerontology" was coined by S. Rattan, and came in regular use with the start of the journal BIOGERONTOLOGY in 2000. It involves interdisciplinary research on the causes, effects, and mechanisms of biological aging. Biogerontologist Leonard Hayflick has said that the natural average lifespan for a human is around 92 years and, if humans do not invent new approaches to treat aging, they will be stuck with this lifespan. James Vaupel has predicted that life expectancy in industrialized countries will reach 100 for children born after the year 2000. Many surveyed biogerontologists have predicted life expectancies of more than three centuries for people born after the year 2100. Other scientists, more controversially, suggest the possibility of unlimited lifespans for those currently living. For example, Aubrey de Grey offers the "tentative timeframe" that with adequate funding of research to develop interventions in aging such as strategies for engineered negligible senescence, "we have a 50/50 chance of developing technology within about 25 to 30 years from now that will, under reasonable assumptions about the rate of subsequent improvements in that technology, allow us to stop people from dying of aging at any age". The idea of this approach is to use presently available technology to extend lifespans of currently living humans long enough for future technological progress to resolve any remaining aging-related issues. This concept has been referred to as longevity escape velocity.
RecQ helicase is a family of helicase enzymes initially found in Escherichia coli that has been shown to be important in genome maintenance. They function through catalyzing the reaction ATP + H2O → ADP + P and thus driving the unwinding of paired DNA and translocating in the 3' to 5' direction. These enzymes can also drive the reaction NTP + H2O → NDP + P to drive the unwinding of either DNA or RNA.

The Hayflick limit, or Hayflick phenomenon, is the number of times a normal somatic, differentiated human cell population will divide before cell division stops. However, this limit does not apply to stem cells.
Enquiry into the evolution of ageing, or aging, aims to explain why a detrimental process such as ageing would evolve, and why there is so much variability in the lifespans of organisms. The classical theories of evolution suggest that environmental factors, such as predation, accidents, disease, and/or starvation, ensure that most organisms living in natural settings will not live until old age, and so there will be very little pressure to conserve genetic changes that increase longevity. Natural selection will instead strongly favor genes which ensure early maturation and rapid reproduction, and the selection for genetic traits which promote molecular and cellular self-maintenance will decline with age for most organisms.
Following is a list of topics related to life extension:
The following outline is provided as an overview of and topical guide to life extension:

An aging-associated disease is a disease that is most often seen with increasing frequency with increasing senescence. They are essentially complications of senescence, distinguished from the aging process itself because all adult animals age but not all adult animals experience all age-associated diseases. The term does not refer to age-specific diseases, such as the childhood diseases chicken pox and measles, only diseases of the elderly. They are also not accelerated aging diseases, all of which are genetic disorders.
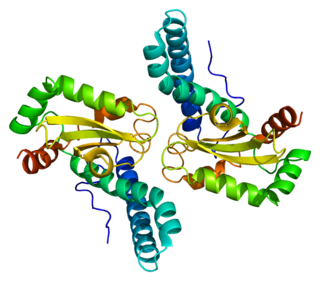
Superoxide dismutase 2, mitochondrial (SOD2), also known as manganese-dependent superoxide dismutase (MnSOD), is an enzyme which in humans is encoded by the SOD2 gene on chromosome 6. A related pseudogene has been identified on chromosome 1. Alternative splicing of this gene results in multiple transcript variants. This gene is a member of the iron/manganese superoxide dismutase family. It encodes a mitochondrial protein that forms a homotetramer and binds one manganese ion per subunit. This protein binds to the superoxide byproducts of oxidative phosphorylation and converts them to hydrogen peroxide and diatomic oxygen. Mutations in this gene have been associated with idiopathic cardiomyopathy (IDC), premature aging, sporadic motor neuron disease, and cancer.
Cellular senescence is a phenomenon characterized by the cessation of cell division. In their experiments during the early 1960s, Leonard Hayflick and Paul Moorhead found that normal human fetal fibroblasts in culture reach a maximum of approximately 50 cell population doublings before becoming senescent. This process is known as "replicative senescence", or the Hayflick limit. Hayflick's discovery of mortal cells paved the path for the discovery and understanding of cellular aging molecular pathways. Cellular senescence can be initiated by a wide variety of stress inducing factors. These stress factors include both environmental and internal damaging events, abnormal cellular growth, oxidative stress, autophagy factors, among many other things.
Ageing is the process of becoming older. The term refers mainly to humans, many other animals, and fungi, whereas for example, bacteria, perennial plants and some simple animals are potentially biologically immortal. In a broader sense, ageing can refer to single cells within an organism which have ceased dividing, or to the population of a species.
The DNA damage theory of aging proposes that aging is a consequence of unrepaired accumulation of naturally occurring DNA damage. Damage in this context is a DNA alteration that has an abnormal structure. Although both mitochondrial and nuclear DNA damage can contribute to aging, nuclear DNA is the main subject of this analysis. Nuclear DNA damage can contribute to aging either indirectly or directly.

The antagonistic pleiotropy hypothesis was first proposed by George C. Williams in 1957 as an evolutionary explanation for senescence. Pleiotropy is the phenomenon where one gene controls more than one phenotypic trait in an organism. A gene is considered to possess antagonistic pleiotropy if it controls more than one trait, where at least one of these traits is beneficial to the organism's fitness early on in life and at least one is detrimental to the organism's fitness later on due to a decline in the force of natural selection. The theme of G.C. William's idea about antagonistic pleiotropy was that if a gene caused both increased reproduction in early life and aging in later life, then senescence would be adaptive in evolution. For example, one study suggests that since follicular depletion in human females causes both more regular cycles in early life and loss of fertility later in life through menopause, it can be selected for by having its early benefits outweigh its late costs.
The stem cell theory of aging postulates that the aging process is the result of the inability of various types of stem cells to continue to replenish the tissues of an organism with functional differentiated cells capable of maintaining that tissue's original function. Damage and error accumulation in genetic material is always a problem for systems regardless of the age. The number of stem cells in young people is very much higher than older people and thus creates a better and more efficient replacement mechanism in the young contrary to the old. In other words, aging is not a matter of the increase in damage, but a matter of failure to replace it due to a decreased number of stem cells. Stem cells decrease in number and tend to lose the ability to differentiate into progenies or lymphoid lineages and myeloid lineages.
David Gems is a British geneticist who studies the biology and genetics of ageing (biogerontology). He is Professor of Biogerontology at the Research Department of Genetics, Evolution and Environment, University College London and he is a co-founder and Research Director of the UCL Institute of Healthy Ageing. His work concerns understanding the underlying causes of aging. His research laboratory tests theories of aging and develops new ones using a short-lived animal model C. elegans.
The disposable soma theory of aging states that organisms age due to an evolutionary trade-off between growth, reproduction, and DNA repair maintenance. Formulated by Thomas Kirkwood, the disposable soma theory explains that an organism only has a limited amount of resources that it can allocate to its various cellular processes. Therefore, a greater investment in growth and reproduction would result in reduced investment in DNA repair maintenance, leading to increased cellular damage, shortened telomeres, accumulation of mutations, compromised stem cells, and ultimately, senescence. Although many models, both animal and human, have appeared to support this theory, parts of it are still controversial. Specifically, while the evolutionary trade-off between growth and aging has been well established, the relationship between reproduction and aging is still without scientific consensus, and the cellular mechanisms largely undiscovered.

The mitochondrial theory of ageing has two varieties: free radical and non-free radical. The first is one of the variants of the free radical theory of ageing. It was formulated by J. Miquel and colleagues in 1980 and was developed in the works of Linnane and coworkers (1989). The second was proposed by A. N. Lobachev in 1978.
Aging is characterized by a progressive loss of physiological integrity, leading to impaired function and increased vulnerability to death. The hallmarks of aging are the types of biochemical changes that occur in all organisms that experience biological aging and lead to a progressive loss of physiological integrity, impaired function and, eventually, death. They were first listed in a landmark paper in 2013 to conceptualize the essence of biological aging and its underlying mechanisms.
References
- ↑ Kowald A, Kirkwood TB. Towards a network theory of ageing: a model combining the free radical theory and the protein error theory J Theor Biol. 1994 May 7;168(1):75-94
- ↑ Promislow DE (Jun 2004). "Protein networks, pleiotropy and the evolution of senescence". Proc Biol Sci. 271 (1545): 1225–34. doi:10.1098/rspb.2004.2732. PMC 1691725 . PMID 15306346.
- ↑ Kriete A, Sokhansanj BA, Coppock DL, West GB (Nov 2006). "Systems approaches to the networks of aging". Ageing Res Rev. 5 (4): 434–48. doi:10.1016/j.arr.2006.06.002. PMID 16904954. S2CID 8220548.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ de Magalhaes JP, Costa J, Toussaint O. HAGR. The Human Ageing Genomic Resources. Nucleic Acids Res. 2005 Jan 1;33(Database issue):D537-43
- 1 2 3 4 5 6 7 8 Simkó, Gábor I.; Gyurkó, Dávid; Veres, Dániel V.; Nánási, Tibor; Csermely, Peter (2009-09-28). "Network strategies to understand the aging process and help age-related drug design". Genome Medicine. 1 (9): 90. arXiv: 0908.4508 . Bibcode:2009arXiv0908.4508S. doi: 10.1186/gm90 . ISSN 1756-994X. PMC 2768997 . PMID 19804610.