Related Research Articles

An axon or nerve fiber is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action potentials away from the nerve cell body. The function of the axon is to transmit information to different neurons, muscles, and glands. In certain sensory neurons, such as those for touch and warmth, the axons are called afferent nerve fibers and the electrical impulse travels along these from the periphery to the cell body and from the cell body to the spinal cord along another branch of the same axon. Axon dysfunction can be the cause of many inherited and acquired neurological disorders that affect both the peripheral and central neurons. Nerve fibers are classed into three types – group A nerve fibers, group B nerve fibers, and group C nerve fibers. Groups A and B are myelinated, and group C are unmyelinated. These groups include both sensory fibers and motor fibers. Another classification groups only the sensory fibers as Type I, Type II, Type III, and Type IV.

Within a nervous system, a neuron, neurone, or nerve cell is an electrically excitable cell that fires electric signals called action potentials across a neural network. Neurons communicate with other cells via synapses, which are specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap.
The following outline is provided as an overview of and topical guide to neuroscience:

Adult neurogenesis is the process in which neurons are generated from neural stem cells in the adult. This process differs from prenatal neurogenesis.

Astrogliosis is an abnormal increase in the number of astrocytes due to the destruction of nearby neurons from central nervous system (CNS) trauma, infection, ischemia, stroke, autoimmune responses or neurodegenerative disease. In healthy neural tissue, astrocytes play critical roles in energy provision, regulation of blood flow, homeostasis of extracellular fluid, homeostasis of ions and transmitters, regulation of synapse function and synaptic remodeling. Astrogliosis changes the molecular expression and morphology of astrocytes, in response to infection for example, in severe cases causing glial scar formation that may inhibit axon regeneration.

A neural circuit is a population of neurons interconnected by synapses to carry out a specific function when activated. Multiple neural circuits interconnect with one another to form large scale brain networks.
In cellular neuroscience, an axotomy is the cutting or otherwise severing of an axon. This type of denervation is often used in experimental studies on neuronal physiology and neuronal death or survival as a method to better understand nervous system diseases.
Neuroplasticity, also known as neural plasticity or brain plasticity, is the ability of neural networks in the brain to change through growth and reorganization. It is when the brain is rewired to function in some way that differs from how it previously functioned. These changes range from individual neuron pathways making new connections, to systematic adjustments like cortical remapping or neural oscillation. Other forms of neuroplasticity include homologous area adaptation, cross modal reassignment, map expansion, and compensatory masquerade. Examples of neuroplasticity include circuit and network changes that result from learning a new ability, information acquisition, environmental influences, practice, and psychological stress.
Neural engineering is a discipline within biomedical engineering that uses engineering techniques to understand, repair, replace, or enhance neural systems. Neural engineers are uniquely qualified to solve design problems at the interface of living neural tissue and non-living constructs.
Gliosis is a nonspecific reactive change of glial cells in response to damage to the central nervous system (CNS). In most cases, gliosis involves the proliferation or hypertrophy of several different types of glial cells, including astrocytes, microglia, and oligodendrocytes. In its most extreme form, the proliferation associated with gliosis leads to the formation of a glial scar.
Neuroconstructivism is a theory that states that phylogenetic developmental processes such as gene–gene interaction, gene–environment interaction and, crucially, ontogeny all play a vital role in how the brain progressively sculpts itself and how it gradually becomes specialized over developmental time.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

The subventricular zone (SVZ) is a region situated on the outside wall of each lateral ventricle of the vertebrate brain. It is present in both the embryonic and adult brain. In embryonic life, the SVZ refers to a secondary proliferative zone containing neural progenitor cells, which divide to produce neurons in the process of neurogenesis. The primary neural stem cells of the brain and spinal cord, termed radial glial cells, instead reside in the ventricular zone (VZ).
Diaschisis is a sudden change of function in a portion of the brain connected to a distant, but damaged, brain area. The site of the originally damaged area and of the diaschisis are connected to each other by neurons. The loss of the damaged structure disrupts the function of the remaining intact systems and causes a physiological imbalance. This can lead both to restitution as well as disruption of distal brain areas. The injury is produced by an acute focal disturbance in an area of the brain, from traumatic brain injury or stroke, for example. Regarding dysfunctional diaschisis, some function may be restored with gradual readjustment of the intact but suppressed areas through intervention and the brain's natural neuroplasticity.
The development of the nervous system in humans, or neural development, or neurodevelopment involves the studies of embryology, developmental biology, and neuroscience. These describe the cellular and molecular mechanisms by which the complex nervous system forms in humans, develops during prenatal development, and continues to develop postnatally.
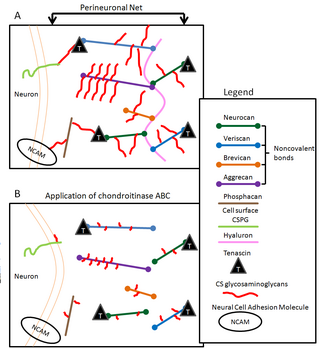
Perineuronal nets (PNNs) are specialized extracellular matrix structures responsible for synaptic stabilization in the adult brain. PNNs are found around certain neuron cell bodies and proximal neurites in the central nervous system. PNNs play a critical role in the closure of the childhood critical period, and their digestion can cause restored critical period-like synaptic plasticity in the adult brain. They are largely negatively charged and composed of chondroitin sulfate proteoglycans, molecules that play a key role in development and plasticity during postnatal development and in the adult.
Activity-dependent plasticity is a form of functional and structural neuroplasticity that arises from the use of cognitive functions and personal experience; hence, it is the biological basis for learning and the formation of new memories. Activity-dependent plasticity is a form of neuroplasticity that arises from intrinsic or endogenous activity, as opposed to forms of neuroplasticity that arise from extrinsic or exogenous factors, such as electrical brain stimulation- or drug-induced neuroplasticity. The brain's ability to remodel itself forms the basis of the brain's capacity to retain memories, improve motor function, and enhance comprehension and speech amongst other things. It is this trait to retain and form memories that is associated with neural plasticity and therefore many of the functions individuals perform on a daily basis. This plasticity occurs as a result of changes in gene expression which are triggered by signaling cascades that are activated by various signaling molecules during increased neuronal activity.
Malleability of intelligence describes the processes by which intelligence can increase or decrease over time and is not static. These changes may come as a result of genetics, pharmacological factors, psychological factors, behavior, or environmental conditions. Malleable intelligence may refer to changes in cognitive skills, memory, reasoning, or muscle memory related motor skills. In general, the majority of changes in human intelligence occur at either the onset of development, during the critical period, or during old age.

Restorative neurology is a branch of neurology dedicated to improving functions of the impaired nervous system through selective structural or functional modification of abnormal neurocontrol according to underlying mechanisms and clinically unrecognized residual functions. When impaired, the body naturally reconstructs new neurological pathways and redirects activity. The field of restorative neurology works to accentuate these new pathways and primarily focuses on the theory of the plasticity of an impaired nervous system. Its main goal is to take a broken down and disordered nervous system and return it to a state of normal function. Certain treatment strategies are used to augment instead of fully replace any performance of surviving and also improving the potential of motor neuron functions. This rehabilitation of motor neurons allows patients a therapeutic approach to recovery opposed to physical structural reconstruction. It is applied in a wide range of disorders of the nervous system, including upper motor neuron dysfunctions like spinal cord injury, cerebral palsy, multiple sclerosis and acquired brain injury including stroke, and neuromuscular diseases as well as for control of pain and spasticity. Instead of applying a reconstructive neurobiological approach, i.e. structural modifications, restorative neurology relies on improving residual function. While subspecialties like neurosurgery and pharmacology exist and are useful in diagnosing and treating conditions of the nervous system, restorative neurology takes a pathophysiological approach. Instead of heavily relying on neurochemistry or perhaps an anatomical discipline, restorative neurology encompasses many fields and blends them together.
Jeffrey D. Macklis is an American neuroscientist. He is the Max and Anne Wien Professor of Life Sciences in the Department of Stem Cell and Regenerative Biology and Center for Brain Science at Harvard University, Professor of Neurology [Neuroscience] at Harvard Medical School, and on the Executive Committee and a Member of the Principal Faculty of the Neuroscience / Nervous System Diseases Program at the Harvard Stem Cell Institute.
References
- ↑ Nilsen, J. (2009), "Synaptic Plasticity: Neuronal Sprouting", Encyclopedia of Neuroscience, Elsevier, pp. 763–767, doi:10.1016/b978-008045046-9.00130-3, ISBN 978-0-08-045046-9 , retrieved 2022-10-31
- ↑ "Neuronal Sprouting Definition". Biology Online. 20 January 2021. Retrieved 2022-10-31.
- ↑ Stroemer, R. Paul; Kent, Thomas A.; Hulsebosch, Claire E. (November 1995). "Neocortical Neural Sprouting, Synaptogenesis, and Behavioral Recovery After Neocortical Infarction in Rats". Stroke. 26 (11): 2135–2144. doi:10.1161/01.STR.26.11.2135. ISSN 0039-2499. PMID 7482662.