
The neutron is a subatomic particle, symbol
n
or
n0
, which has a neutral charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, they are both referred to as nucleons. Nucleons have a mass of approximately one atomic mass unit, or dalton. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks.
In physical cosmology, Big Bang nucleosynthesis is the production of nuclei other than those of the lightest isotope of hydrogen during the early phases of the universe. This type of nucleosynthesis is thought by most cosmologists to have occurred from 10 seconds to 20 minutes after the Big Bang. It is thought to be responsible for the formation of most of the universe's helium, along with small fractions of the hydrogen isotope deuterium, the helium isotope helium-3 (3He), and a very small fraction of the lithium isotope lithium-7 (7Li). In addition to these stable nuclei, two unstable or radioactive isotopes were produced: the heavy hydrogen isotope tritium and the beryllium isotope beryllium-7 (7Be). These unstable isotopes later decayed into 3He and 7Li, respectively, as above.

Oganesson is a synthetic chemical element; it has symbol Og and atomic number 118. It was first synthesized in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, near Moscow, Russia, by a joint team of Russian and American scientists. In December 2015, it was recognized as one of four new elements by the Joint Working Party of the international scientific bodies IUPAC and IUPAP. It was formally named on 28 November 2016. The name honors the nuclear physicist Yuri Oganessian, who played a leading role in the discovery of the heaviest elements in the periodic table. It is one of only two elements named after a person who was alive at the time of naming, the other being seaborgium, and the only element whose eponym is alive as of 2024.

In nuclear physics, the island of stability is a predicted set of isotopes of superheavy elements that may have considerably longer half-lives than known isotopes of these elements. It is predicted to appear as an "island" in the chart of nuclides, separated from known stable and long-lived primordial radionuclides. Its theoretical existence is attributed to stabilizing effects of predicted "magic numbers" of protons and neutrons in the superheavy mass region.

Unbinilium, also known as eka-radium or element 120, is a hypothetical chemical element; it has symbol Ubn and atomic number 120. Unbinilium and Ubn are the temporary systematic IUPAC name and symbol, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be an s-block element, an alkaline earth metal, and the second element in the eighth period. It has attracted attention because of some predictions that it may be in the island of stability.
Flerovium is a superheavy synthetic chemical element; it has symbol Fl and atomic number 114. It is an extremely radioactive synthetic element, named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Dubna, Russia, where the element was discovered in 1999. The lab's name, in turn, honours Russian physicist Georgy Flyorov. IUPAC adopted the name on 30 May 2012. The name and symbol had previously been proposed for element 102 (nobelium), but was not accepted by IUPAC at that time.
Tetraneutron is considered an unbound isotope with a lifetime around 10-22 seconds. The stability of this cluster of four neutrons is not supported by current models of nuclear forces. Recent empirical evidence is "consistent with a quasi-bound tetraneutron state existing for a very short time".

In nuclear physics, a magic number is a number of nucleons such that they are arranged into complete shells within the atomic nucleus. As a result, atomic nuclei with a "magic" number of protons or neutrons are much more stable than other nuclei. The seven most widely recognized magic numbers as of 2019 are 2, 8, 20, 28, 50, 82, and 126.
A hypernucleus is similar to a conventional atomic nucleus, but contains at least one hyperon in addition to the normal protons and neutrons. Hyperons are a category of baryon particles that carry non-zero strangeness quantum number, which is conserved by the strong and electromagnetic interactions.

In nuclear physics, an atomic nucleus is called a halo nucleus or is said to have a nuclear halo when it has a core nucleus surrounded by a "halo" of orbiting protons or neutrons, which makes the radius of the nucleus appreciably larger than that predicted by the liquid drop model. Halo nuclei form at the extreme edges of the table of nuclides — the neutron drip line and proton drip line — and have short half-lives, measured in milliseconds. These nuclei are studied shortly after their formation in an ion beam.
Flerovium (114Fl) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 289Fl in 1999. Flerovium has six known isotopes, along with the unconfirmed 290Fl, and possibly two nuclear isomers. The longest-lived isotope is 289Fl with a half-life of 1.9 seconds, but 290Fl may have a longer half-life of 19 seconds.

Calcium-48 is a scarce isotope of calcium containing 20 protons and 28 neutrons. It makes up 0.187% of natural calcium by mole fraction. Although it is unusually neutron-rich for such a light nucleus, its beta decay is extremely hindered, and so the only radioactive decay pathway that it has been observed to undergo is the extremely rare process of double beta decay. Its half-life is about 6.4×1019 years, so for all practical purposes it can be treated as stable. One factor contributing to this unusual stability is that 20 and 28 are both magic numbers, making 48Ca a "doubly magic" nucleus.

Nuclear matter is an idealized system of interacting nucleons that exists in several phases of exotic matter that, as of yet, are not fully established. It is not matter in an atomic nucleus, but a hypothetical substance consisting of a huge number of protons and neutrons held together by only nuclear forces and no Coulomb forces. Volume and the number of particles are infinite, but the ratio is finite. Infinite volume implies no surface effects and translational invariance.
In particle physics, hexaquarks, alternatively known as sexaquarks, are a large family of hypothetical particles, each particle consisting of six quarks or antiquarks of any flavours. Six constituent quarks in any of several combinations could yield a colour charge of zero; for example a hexaquark might contain either six quarks, resembling two baryons bound together, or three quarks and three antiquarks. Once formed, dibaryons are predicted to be fairly stable by the standards of particle physics.
A strangelet is a hypothetical particle consisting of a bound state of roughly equal numbers of up, down, and strange quarks. An equivalent description is that a strangelet is a small fragment of strange matter, small enough to be considered a particle. The size of an object composed of strange matter could, theoretically, range from a few femtometers across to arbitrarily large. Once the size becomes macroscopic, such an object is usually called a strange star. The term "strangelet" originates with Edward Farhi and Robert Jaffe in 1984. Strangelets can convert matter to strange matter on contact. Strangelets have been suggested as a dark matter candidate.
The EMC effect is the surprising observation that the cross section for deep inelastic scattering from an atomic nucleus is different from that of the same number of free protons and neutrons. From this observation, it can be inferred that the quark momentum distributions in nucleons bound inside nuclei are different from those of free nucleons. This effect was first observed in 1983 at CERN by the European Muon Collaboration, hence the name "EMC effect". It was unexpected, since the average binding energy of protons and neutrons inside nuclei is insignificant when compared to the energy transferred in deep inelastic scattering reactions that probe quark distributions. While over 1000 scientific papers have been written on the topic and numerous hypotheses have been proposed, no definitive explanation for the cause of the effect has been confirmed. Determining the origin of the EMC effect is one of the major unsolved problems in the field of nuclear physics.

The nuclear drip line is the boundary beyond which atomic nuclei are unbound with respect to the emission of a proton or neutron.
The rms charge radius is a measure of the size of an atomic nucleus, particularly the proton distribution. The proton radius is about one femtometre = 10−15 metre. It can be measured by the scattering of electrons by the nucleus. Relative changes in the mean squared nuclear charge distribution can be precisely measured with atomic spectroscopy.

A Borromean nucleus is an atomic nucleus comprising three bound components in which any subsystem of two components is unbound. This has the consequence that if one component is removed, the remaining two comprise an unbound resonance, so that the original nucleus is split into three parts.
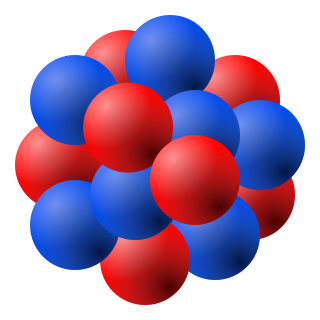
The shape of the atomic nucleus been depicted as a compact bundle of the two types of nucleons that look like little balls stuck together, protons (red) and neutrons (blue). This depiction of the atomic nucleus approximates the empirical evidence for the size and shape of nucleons and nuclei as outlined in the article below, beginning with the discovery of the quadrapole moment in 1935 and its role in shape. Factors affecting nuclear shape include the prolate spheroid shape of the nucleon, the distance between nucleons, and the radial charge density distribution. The unusual cosmic abundance of alpha nuclides has inspired geometric arrangements of alpha particles as a solution to nuclear shapes, although the atomic nucleus generally assumes a prolate spheroid shape. Nuclides can also be discus-shaped, triaxial or pear-shaped.








